Noninvasive in vivo cell tracking using molecular imaging: A useful tool for developing mesenchymal stem cell-based cancer treatment
- PMID: 33505597
- PMCID: PMC7789123
- DOI: 10.4252/wjsc.v12.i12.1492
Noninvasive in vivo cell tracking using molecular imaging: A useful tool for developing mesenchymal stem cell-based cancer treatment
Abstract
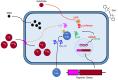
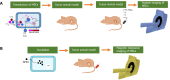
Mounting evidence has emphasized the potential of cell therapies in treating various diseases by restoring damaged tissues or replacing defective cells in the body. Cell therapies have become a strong therapeutic modality by applying noninvasive in vivo molecular imaging for examining complex cellular processes, understanding pathophysiological mechanisms of diseases, and evaluating the kinetics/dynamics of cell therapies. In particular, mesenchymal stem cells (MSCs) have shown promise in recent years as drug carriers for cancer treatment. They can also be labeled with different probes and tracked in vivo to assess the in vivo effect of administered cells, and to optimize therapy. The exact role of MSCs in oncologic diseases is not clear as MSCs have been shown to be involved in tumor progression and inhibition, and the exact interactions between MSCs and specific cancer microenvironments are not clear. In this review, a multitude of labeling approaches, imaging modalities, and the merits/demerits of each strategy are outlined. In addition, specific examples of the use of MSCs and in vivo imaging in cancer therapy are provided. Finally, present limitations and future outlooks in terms of the translation of different imaging approaches in clinics are discussed.
Keywords: Cell therapy; Drug delivery; In vivo molecular imaging; Mesenchymal stem cells; Superparamagnetic iron oxide.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that the research was performed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
In vivo magnetic resonance imaging of iron oxide-labeled, intravenous-injected mesenchymal stem cells in kidneys of rabbits with acute ischemic kidney injury: detection and monitoring at 1.5 T.Ren Fail. 2015;37(8):1363-9. doi: 10.3109/0886022x.2015.1073542. Epub 2015 Aug 7. Ren Fail. 2015. PMID: 26248484
-
MRI tracking of polyethylene glycol-coated superparamagnetic iron oxide-labelled placenta-derived mesenchymal stem cells toward glioblastoma stem-like cells in a mouse model.Artif Cells Nanomed Biotechnol. 2018;46(sup3):S448-S459. doi: 10.1080/21691401.2018.1499661. Epub 2018 Sep 8. Artif Cells Nanomed Biotechnol. 2018. PMID: 30198338
-
Human Umbilical Cord Wharton's Jelly-Derived Mesenchymal Stem Cells Labeled with Mn2+ and Gd3+ Co-Doped CuInS2-ZnS Nanocrystals for Multimodality Imaging in a Tumor Mice Model.ACS Appl Mater Interfaces. 2020 Jan 22;12(3):3415-3429. doi: 10.1021/acsami.9b19054. Epub 2020 Jan 9. ACS Appl Mater Interfaces. 2020. PMID: 31875453
-
Tracking mesenchymal stem cells using magnetic resonance imaging.Brain Circ. 2016 Jul-Sep;2(3):108-113. doi: 10.4103/2394-8108.192521. Epub 2016 Oct 18. Brain Circ. 2016. PMID: 30276283 Free PMC article. Review.
-
Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases.Expert Opin Biol Ther. 2005 Dec;5(12):1571-84. doi: 10.1517/14712598.5.12.1571. Expert Opin Biol Ther. 2005. PMID: 16318421 Free PMC article. Review.
Cited by
-
Iron Oxide Nanoparticles in Mesenchymal Stem Cell Detection and Therapy.Stem Cell Rev Rep. 2022 Oct;18(7):2234-2261. doi: 10.1007/s12015-022-10343-x. Epub 2022 Feb 1. Stem Cell Rev Rep. 2022. PMID: 35103937 Free PMC article. Review.
-
Tracking of Oral and Craniofacial Stem Cells in Tissue Development, Regeneration, and Diseases.Curr Osteoporos Rep. 2021 Dec;19(6):656-668. doi: 10.1007/s11914-021-00705-8. Epub 2021 Nov 6. Curr Osteoporos Rep. 2021. PMID: 34741728 Review.
-
Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy.Pharmaceuticals (Basel). 2021 Dec 22;15(1):13. doi: 10.3390/ph15010013. Pharmaceuticals (Basel). 2021. PMID: 35056071 Free PMC article. Review.
-
Optimal Intravenous Administration Procedure for Efficient Delivery of Canine Adipose-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2022 Nov 24;23(23):14681. doi: 10.3390/ijms232314681. Int J Mol Sci. 2022. PMID: 36499004 Free PMC article.
-
IONPs-Based Medical Imaging in Cancer Care: Moving Beyond Traditional Diagnosis and Therapeutic Assessment.Int J Nanomedicine. 2023 Apr 1;18:1741-1763. doi: 10.2147/IJN.S399047. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37034271 Free PMC article. Review.
References
-
- Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med. 2014;9:27–39. - PubMed
-
- von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

