Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine
- PMID: 33505599
- PMCID: PMC7789121
- DOI: 10.4252/wjsc.v12.i12.1529
Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine
Abstract
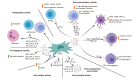
Mesenchymal stem cells (MSCs) are the most frequently used stem cells in clinical trials due to their easy isolation from various adult tissues, their ability of homing to injury sites and their potential to differentiate into multiple cell types. However, the realization that the beneficial effect of MSCs relies mainly on their paracrine action, rather than on their engraftment in the recipient tissue and subsequent differentiation, has opened the way to cell-free therapeutic strategies in regenerative medicine. All the soluble factors and vesicles secreted by MSCs are commonly known as secretome. MSCs secretome has a key role in cell-to-cell communication and has been proven to be an active mediator of immune-modulation and regeneration both in vitro and in vivo. Moreover, the use of secretome has key advantages over cell-based therapies, such as a lower immunogenicity and easy production, handling and storage. Importantly, MSCs can be modulated to alter their secretome composition to better suit specific therapeutic goals, thus, opening a large number of possibilities. Altogether these advantages now place MSCs secretome at the center of an important number of investigations in different clinical contexts, enabling rapid scientific progress in this field.
Keywords: Bone regeneration; Exosomes; Extra-cellular vesicles; Mesenchymal stem cells; Secretome; Soluble factors.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: None of the authors have any conflicts of interest relevant to this study.
Figures
References
-
- Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA. Angiogenic and Restorative Abilities of Human Mesenchymal Stem Cells Were Reduced Following Treatment With Serum From Diabetes Mellitus Type 2 Patients. J Cell Biochem. 2018;119:524–535. - PubMed
-
- Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, Büscher D, Fibbe W, Foussat A, Kwa M, Lantz O, Mačiulaitis R, Palomäki T, Schneider CK, Sensebé L, Tachdjian G, Tarte K, Tosca L, Salmikangas P. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753–759. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

