Determination of Pharmaceutical Residues by UPLC-MS/MS Method: Validation and Application on Surface Water and Hospital Wastewater
- PMID: 33505763
- PMCID: PMC7811430
- DOI: 10.1155/2021/6628285
Determination of Pharmaceutical Residues by UPLC-MS/MS Method: Validation and Application on Surface Water and Hospital Wastewater
Abstract
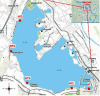
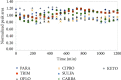
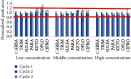
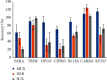
In this study, an analytical method for the simultaneous determination of 7 major pharmaceutical residues in Vietnam, namely, carbamazepine, ciprofloxacin, ofloxacin, ketoprofen, paracetamol, sulfamethoxazole, and trimethoprim, in surface water and hospital wastewater has been developed. The method includes enrichment and clean-up steps by solid phase extraction using mix-mode cation exchange, followed by identification and quantification using an ultrahigh-performance liquid chromatography and tandem mass spectrometry and employing electrospray ionization (UPLC-ESI-MS/MS). Seven target compounds were separated on the reversed phase column and detected in multiple reaction monitoring (MRM) mode within 6 minutes. The present study also optimized the operating parameters of the mass spectrometer to achieve the highest analytical signals for all target compounds. All characteristic parameters of the analytical method were investigated, including linearity range, limit of detection, limit of quantification, precision, and accuracy. The important parameter in UPLC-ESI-MS/MS, matrix effect, was assessed and implemented via preextraction and postextraction spiking experiments. The overall recoveries of all target compounds were in the ranges from 55% to 109% and 56 % to 115% for surface water and hospital wastewater, respectively. Detection limits for surface water and hospital wastewater were 0.005-0.015 µg L-1 and 0.014-0.123 µg L-1, respectively. The sensitivity of the developed method was allowed for determination of target compounds at trace level in environmental water samples. The in-house validation of the developed method was performed by spiking experiment in both the surface water and hospital wastewater matrix. The method was then applied to analyze several surface water and hospital wastewater samples taken from West Lake and some hospitals in Vietnam, where the level of these pharmaceutical product residues was still missed. Sulfamethoxazole was present at a high detection frequency in both surface water (33% of analyzed samples) and hospital wastewater (81% of analyzed samples) samples.
Copyright © 2021 Bui Van Hoi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Multiresidue methods for the analysis of pharmaceuticals, personal care products and illicit drugs in surface water and wastewater by solid-phase extraction and ultra performance liquid chromatography-electrospray tandem mass spectrometry.Anal Bioanal Chem. 2008 Jun;391(4):1293-308. doi: 10.1007/s00216-008-1854-x. Epub 2008 Feb 6. Anal Bioanal Chem. 2008. PMID: 18253724
-
Simultaneous ultra-high-pressure liquid chromatography-tandem mass spectrometry determination of amphetamine and amphetamine-like stimulants, cocaine and its metabolites, and a cannabis metabolite in surface water and urban wastewater.J Chromatogr A. 2009 Apr 10;1216(15):3078-89. doi: 10.1016/j.chroma.2009.01.067. Epub 2009 Jan 29. J Chromatogr A. 2009. PMID: 19201418
-
MCX based solid phase extraction combined with liquid chromatography tandem mass spectrometry for the simultaneous determination of 31 endocrine-disrupting compounds in surface water of Shanghai.J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Oct 15;879(28):2998-3004. doi: 10.1016/j.jchromb.2011.08.036. Epub 2011 Sep 3. J Chromatogr B Analyt Technol Biomed Life Sci. 2011. PMID: 21930438
-
Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Oct 15;969:162-70. doi: 10.1016/j.jchromb.2014.08.008. Epub 2014 Aug 12. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 25171505
-
Development of a new multi-residue laser diode thermal desorption atmospheric pressure chemical ionization tandem mass spectrometry method for the detection and quantification of pesticides and pharmaceuticals in wastewater samples.Anal Chim Acta. 2012 Nov 19;754:75-82. doi: 10.1016/j.aca.2012.09.046. Epub 2012 Oct 17. Anal Chim Acta. 2012. PMID: 23140957
Cited by
-
Development and Validation of UFLC-MS/MS Analytical Method for the Simultaneous Quantification of Antibiotic Residues in Surface Water, Groundwater, and Pharmaceutical Waste Water Samples from South India.ACS Omega. 2024 Mar 6;9(11):12801-12809. doi: 10.1021/acsomega.3c08566. eCollection 2024 Mar 19. ACS Omega. 2024. PMID: 38524455 Free PMC article.
-
Responses of Aroma Related Metabolic Attributes of Opisthopappus longilobus Flowers to Environmental Changes.Plants (Basel). 2023 Apr 10;12(8):1592. doi: 10.3390/plants12081592. Plants (Basel). 2023. PMID: 37111816 Free PMC article.
-
Development of a Colloidal Gold Immunochromatographic Assay Strip Using a Monoclonal Antibody for the Rapid Detection of Ofloxacin.Foods. 2024 Dec 20;13(24):4137. doi: 10.3390/foods13244137. Foods. 2024. PMID: 39767079 Free PMC article.
References
-
- Daughton C. G. Pharmaceuticals in the Environment: Sources and Their Management. 2nd. Vol. 62. Amsterdam, Netherlands: Elsevier B. V.; 2013.
-
- Dogan A., Płotka-Wasylka J., Kempińska-Kupczyk D., Namieśnik J., Kot-Wasik A. Detection, identification and determination of chiral pharmaceutical residues in wastewater: problems and challenges. TrAC Trends in Analytical Chemistry. 2020;122:p. 115710. doi: 10.1016/j.trac.2019.115710. - DOI
-
- López-Serna R., Marín-de-Jesús D., Irusta-Mata R., et al. Multiresidue analytical method for pharmaceuticals and personal care products in sewage and sewage sludge by online direct immersion SPME on-fiber derivatization-GCMS. Talanta. 2018;186:506–512. doi: 10.1016/j.talanta.2018.04.099. - DOI - PubMed
-
- Tan E. S. S., Ho Y. B., Zakaria M. P., Latif P. A., Saari N. Simultaneous extraction and determination of pharmaceuticals and personal care products (PPCPs) in river water and sewage by solid-phase extraction and liquid chromatography-tandem mass spectrometry. International Journal of Environmental Analytical Chemistry. 2015;95(9):1–17. doi: 10.1080/03067319.2015.1058929. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

