Biochemical characterization and inhibition of thermolabile hemolysin from Vibrio parahaemolyticus by phenolic compounds
- PMID: 33505784
- PMCID: PMC7796666
- DOI: 10.7717/peerj.10506
Biochemical characterization and inhibition of thermolabile hemolysin from Vibrio parahaemolyticus by phenolic compounds
Abstract
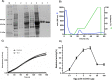
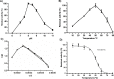
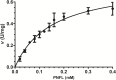
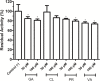
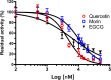
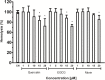
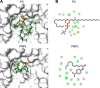
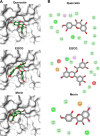
Vibrio parahaemolyticus (Vp), a typical microorganism inhabiting marine ecosystems, uses pathogenic virulence molecules such as hemolysins to cause bacterial infections of both human and marine animals. The thermolabile hemolysin VpTLH lyses human erythrocytes by a phospholipase B/A2 enzymatic activity in egg-yolk lecithin. However, few studies have been characterized the biochemical properties and the use of VpTLH as a molecular target for natural compounds as an alternative to control Vp infection. Here, we evaluated the biochemical and inhibition parameters of the recombinant VpTLH using enzymatic and hemolytic assays and determined the molecular interactions by in silico docking analysis. The highest enzymatic activity was at pH 8 and 50 °C, and it was inactivated by 20 min at 60 °C with Tm = 50.9 °C. Additionally, the flavonoids quercetin, epigallocatechin gallate, and morin inhibited the VpTLH activity with IC50 values of 4.5 µM, 6.3 µM, and 9.9 µM, respectively; while phenolics acids were not effective inhibitors for this enzyme. Boltzmann and Arrhenius equation analysis indicate that VpTLH is a thermolabile enzyme. The inhibition of both enzymatic and hemolytic activities by flavonoids agrees with molecular docking, suggesting that flavonoids could interact with the active site's amino acids. Future research is necessary to evaluate the antibacterial activity of flavonoids against Vp in vivo.
Keywords: Inhibition; Molecular docking; Phenolic compounds; SGHN phospholipases; Thermal stability; Thermolabile-hemolysin.
©2021 Vazquez-Morado et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
Purification and Characterization of the Lecithin-Dependent Thermolabile Hemolysin Vhe1 from the Vibrio sp. Strain MA3 Associated with Mass Mortality of Pearl Oyster (Pinctada fucata).Curr Microbiol. 2023 Jul 17;80(9):288. doi: 10.1007/s00284-023-03409-7. Curr Microbiol. 2023. PMID: 37458864
-
Expression, purification, and characterization of thermolabile hemolysin (TLH) from Vibrio alginolyticus.Dis Aquat Organ. 2010 Jun 11;90(2):121-7. doi: 10.3354/dao02225. Dis Aquat Organ. 2010. PMID: 20662368
-
[The comparative characteristics of Vibrio cholerae hemolysins].Mikrobiol Zh (1978). 1992 Sep-Oct;54(5):105-12. Mikrobiol Zh (1978). 1992. PMID: 1453988 Review. Russian.
-
Partial purification and characterization of hemolysin from a psychrotrophic kanagawa-positive marine Vibrio.Appl Environ Microbiol. 1982 Jan;43(1):39-49. doi: 10.1128/aem.43.1.39-49.1982. Appl Environ Microbiol. 1982. PMID: 16345927 Free PMC article.
-
Molecular mechanisms of Vibrio parahaemolyticus pathogenesis.Microbiol Res. 2019 May;222:43-51. doi: 10.1016/j.micres.2019.03.003. Epub 2019 Mar 8. Microbiol Res. 2019. PMID: 30928029 Review.
Cited by
-
Catalytic site flexibility facilitates the substrate and catalytic promiscuity of Vibrio dual lipase/transferase.Nat Commun. 2023 Aug 9;14(1):4795. doi: 10.1038/s41467-023-40455-y. Nat Commun. 2023. PMID: 37558668 Free PMC article.
-
Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties.Materials (Basel). 2022 Jan 19;15(3):748. doi: 10.3390/ma15030748. Materials (Basel). 2022. PMID: 35160695 Free PMC article.
-
Exploring the Venom Gland Transcriptome of Bothrops asper and Bothrops jararaca: De Novo Assembly and Analysis of Novel Toxic Proteins.Toxins (Basel). 2024 Nov 27;16(12):511. doi: 10.3390/toxins16120511. Toxins (Basel). 2024. PMID: 39728769 Free PMC article.
-
Study of the Chemical Composition and Biologically Active Properties of Glycyrrhiza glabra Extracts.Life (Basel). 2022 Nov 2;12(11):1772. doi: 10.3390/life12111772. Life (Basel). 2022. PMID: 36362927 Free PMC article.
-
In Vitro Study of Biological Activity of Tanacetum vulgare Extracts.Pharmaceutics. 2023 Feb 12;15(2):616. doi: 10.3390/pharmaceutics15020616. Pharmaceutics. 2023. PMID: 36839938 Free PMC article.
References
-
- Bej AK, Patterson DP, Brasher CW, Vickery MC, Jones DD, Kaysner CA. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. Journal of Microbiological Methods. 1999;36:215–225. doi: 10.1016/s0167-7012(99)00037-8. - DOI - PubMed
-
- Cheynier V. Phenolic compounds: from plants to foods. Phytochemistry Reviews. 2012;11:153–177. doi: 10.1007/s11101-012-9242-8. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

