Is hematopoietic stem cell transplantation a therapeutic option for mucolipidosis type II?
- PMID: 33505859
- PMCID: PMC7815485
- DOI: 10.1016/j.ymgmr.2020.100704
Is hematopoietic stem cell transplantation a therapeutic option for mucolipidosis type II?
Abstract
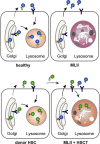
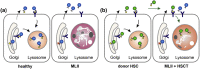
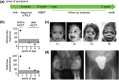
Background: Mucolipidosis type II (MLII) is an ultra-rare lysosomal storage disorder caused by defective lysosomal enzyme trafficking. Clinical hallmarks are craniofacial dysmorphia, cardiorespiratory dysfunction, hepatosplenomegaly, skeletal deformities and neurocognitive retardation. Death usually occurs in the first decade of life and no cure exists. Hematopoietic stem cell transplantation (HSCT) has been performed in few MLII patients, but comprehensive follow-up data are extremely scarce.
Methods: MLII diagnosis was confirmed in a female three-month-old patient with the mutations c.2213C > A and c.2220_2221dup in the GNPTAB gene. At nine months of age, the patient received HSCT from a 9/10 human leukocyte antigen (HLA)-matched unrelated donor.
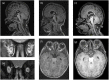
Results: HSCT resulted in a sustained reduction of lysosomal storage und bone metabolism markers. At six years of age, the patient showed normal cardiac function, partial respiratory insufficiency and moderate hepatomegaly, whereas skeletal manifestations had progressed. However, the patient could walk and maintained an overall good quality of life. Neurocognitive testing revealed a developmental quotient of 36%. The patient died at 6.6 years of age following a human metapneumovirus (hMPV) pneumonia.
Conclusions: The exact benefit remains unclear as current literature vastly lacks comparable data on MLII natural history patients. In order to evaluate experimental therapies, in-depth prospective studies and registries of untreated MLII patients are indispensable.
Keywords: Bone marrow cell transplantation; Cognitive function; Hematopoietic stem cell transplantation; I-cell disease; Life quality; Lysosomal storage disorder; Mucolipidosis type II; Treatment.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Outcomes after HSCT for mucolipidosis II (I-cell disease) caused by novel compound heterozygous GNPTAB mutations.Front Pediatr. 2023 Jul 6;11:1199489. doi: 10.3389/fped.2023.1199489. eCollection 2023. Front Pediatr. 2023. PMID: 37484777 Free PMC article.
-
CNS Manifestations in Mucolipidosis Type II-A Retrospective Analysis of Longitudinal Data on Neurocognitive Development and Neuroimaging in Eleven Patients.J Clin Med. 2023 Jun 18;12(12):4114. doi: 10.3390/jcm12124114. J Clin Med. 2023. PMID: 37373807 Free PMC article.
-
Outcomes after hematopoietic stem cell transplantation for children with I-cell disease.Biol Blood Marrow Transplant. 2014 Nov;20(11):1847-51. doi: 10.1016/j.bbmt.2014.06.019. Epub 2014 Jul 10. Biol Blood Marrow Transplant. 2014. PMID: 25016194 Free PMC article.
-
Mucolipidosis type II and type III: a systematic review of 843 published cases.Genet Med. 2021 Nov;23(11):2047-2056. doi: 10.1038/s41436-021-01244-4. Epub 2021 Jun 25. Genet Med. 2021. PMID: 34172897
-
[Treatment of Gaucher disease with allogeneic hematopoietic stem cell transplantation: report of three cases and review of literatures].Zhonghua Er Ke Za Zhi. 2015 Nov;53(11):810-6. Zhonghua Er Ke Za Zhi. 2015. PMID: 26758318 Review. Chinese.
Cited by
-
Anaesthesia-Relevant Disease Manifestations and Perianaesthetic Complications in Patients with Mucolipidosis-A Retrospective Analysis of 44 Anaesthetic Cases in 12 Patients.J Clin Med. 2022 Jun 24;11(13):3650. doi: 10.3390/jcm11133650. J Clin Med. 2022. PMID: 35806935 Free PMC article.
-
Outcomes after HSCT for mucolipidosis II (I-cell disease) caused by novel compound heterozygous GNPTAB mutations.Front Pediatr. 2023 Jul 6;11:1199489. doi: 10.3389/fped.2023.1199489. eCollection 2023. Front Pediatr. 2023. PMID: 37484777 Free PMC article.
-
CNS Manifestations in Mucolipidosis Type II-A Retrospective Analysis of Longitudinal Data on Neurocognitive Development and Neuroimaging in Eleven Patients.J Clin Med. 2023 Jun 18;12(12):4114. doi: 10.3390/jcm12124114. J Clin Med. 2023. PMID: 37373807 Free PMC article.
-
Case Report: Mucolipidosis II and III Alpha/Beta Caused by Pathogenic Variants in the GNPTAB Gene (Mucolipidosis).Front Pediatr. 2022 Apr 8;10:852701. doi: 10.3389/fped.2022.852701. eCollection 2022. Front Pediatr. 2022. PMID: 35463894 Free PMC article.
-
Pathogenic variants in GNPTAB and GNPTG encoding distinct subunits of GlcNAc-1-phosphotransferase differentially impact bone resorption in patients with mucolipidosis type II and III.Genet Med. 2021 Dec;23(12):2369-2377. doi: 10.1038/s41436-021-01285-9. Epub 2021 Aug 2. Genet Med. 2021. PMID: 34341521 Free PMC article.
References
-
- Velho R.V., Harms F.L., Danyukova T., Ludwig N.F., Friez M.J., Cathey S.S., Filocamo M., Tappino B., Gunes N., Tuysuz B., Tylee K.L., Brammeier K.L., Heptinstall L., Oussoren E., van der Ploeg A.T., Petersen C., Alves S., Saavedra G.D., Schwartz I.V., Muschol N., Kutsche K., Pohl S. The lysosomal storage disorders mucolipidosis type II, type III alpha/beta, and type III gamma: Update on GNPTAB and GNPTG mutations. Hum. Mutat. 2019;40:842–864. - PubMed
-
- Hoogerbrugge P.M., Brouwer O.F., Bordigoni P., Ringden O., Kapaun P., Ortega J.J., O'Meara A., Cornu G., Souillet G., Frappaz D. Allogeneic bone marrow transplantation for lysosomal storage diseases. Eur. Group Bone Marrow Transp. Lancet. 1995;345:1398–1402. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

