Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients With Colon Cancer: A Clinical Intervention Study
- PMID: 33505915
- PMCID: PMC7830882
- DOI: 10.3389/fonc.2020.600715
Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients With Colon Cancer: A Clinical Intervention Study
Abstract
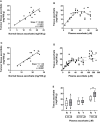
The use of high dose ascorbate infusions in cancer patients is widespread, but without evidence of efficacy. Several mechanisms whereby ascorbate could affect tumor progression have been proposed, including: (i) the localized generation of cytotoxic quantities of H2O2; (ii) ascorbate-dependent activation of the 2-oxoglutarate-dependent dioxygenases that control the hypoxia-inducible factors (HIFs) and that are responsible for the demethylation of DNA and histones; (iii) increased oxidative stress induced by dehydroascorbic acid. We hypothesize that the dysfunctional vasculature of solid tumors results in compromised delivery of ascorbate to poorly perfused regions of the tumor and that this ascorbate deficit acts as an additional driver of the hypoxic response via upregulation of HIFs. Using a randomized "therapeutic window of opportunity" clinical study design we aimed to determine whether ascorbate infusions affected tumor ascorbate content and tumor biology. Patients with colon cancer were randomized to receive infusions of up to 1 g/kg ascorbate for 4 days before surgical resection (n = 9) or to not receive infusions (n = 6). Ascorbate was measured in plasma, erythrocytes, tumor and histologically normal mucosa at diagnostic colonoscopy and at surgery. Protein markers of tumor hypoxia or DNA damage were monitored in resected tissue. Plasma ascorbate reached millimolar levels following infusion and returned to micromolar levels over 24 h. Pre-infusion plasma ascorbate increased from 38 ± 10 µM to 241 ± 33 µM (p < 0.0001) over 4 days and erythrocyte ascorbate from 18 ± 20 µM to 2509 ± 1016 µM (p < 0.005). Tumor ascorbate increased from 15 ± 6 to 28 ± 6 mg/100 g tissue (p < 0.0001) and normal tissue from 14 ± 6 to 21 ± 4 mg/100 g (p < 0.001). A gradient of lower ascorbate was evident towards the tumor centre in both control and infusion samples. Lower expression of hypoxia-associated proteins was seen in post-infusion tumors compared with controls. There were no significant adverse events and quality of life was unaffected by ascorbate infusion. This is the first clinical study to demonstrate that tumor ascorbate levels increase following infusion, even in regions of poor diffusion, and that this could modify tumor biology.
Clinical trial registration: ANZCTR Trial ID ACTRN12615001277538 (https://www.anzctr.org.au/).
Keywords: colorectal cancer; hypoxia-inducible factor; normal mucosa; plasma; tumor; tumor hypoxia; vitamin C.
Copyright © 2021 Dachs, Gandhi, Wohlrab, Carr, Morrin, Pullar, Bayer, Eglinton, Robinson and Vissers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Peroxiredoxin 2 oxidation reveals hydrogen peroxide generation within erythrocytes during high-dose vitamin C administration.Redox Biol. 2021 Jul;43:101980. doi: 10.1016/j.redox.2021.101980. Epub 2021 Apr 17. Redox Biol. 2021. PMID: 33905956 Free PMC article.
-
Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue.Free Radic Biol Med. 2014 Dec;77:340-52. doi: 10.1016/j.freeradbiomed.2014.09.023. Epub 2014 Sep 30. Free Radic Biol Med. 2014. PMID: 25277418
-
Increased Tumor Ascorbate is Associated with Extended Disease-Free Survival and Decreased Hypoxia-Inducible Factor-1 Activation in Human Colorectal Cancer.Front Oncol. 2014 Feb 4;4:10. doi: 10.3389/fonc.2014.00010. eCollection 2014. Front Oncol. 2014. PMID: 24551593 Free PMC article.
-
Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression.Front Oncol. 2014 Dec 10;4:359. doi: 10.3389/fonc.2014.00359. eCollection 2014. Front Oncol. 2014. PMID: 25540771 Free PMC article. Review.
-
Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence.Front Physiol. 2018 Jul 3;9:809. doi: 10.3389/fphys.2018.00809. eCollection 2018. Front Physiol. 2018. PMID: 30018566 Free PMC article. Review.
Cited by
-
The Membrane Electrical Potential and Intracellular pH as Factors Influencing Intracellular Ascorbate Concentration and Their Role in Cancer Treatment.Cells. 2021 Oct 30;10(11):2964. doi: 10.3390/cells10112964. Cells. 2021. PMID: 34831187 Free PMC article.
-
Overcoming EGFR Resistance in Metastatic Colorectal Cancer Using Vitamin C: A Review.Biomedicines. 2023 Feb 23;11(3):678. doi: 10.3390/biomedicines11030678. Biomedicines. 2023. PMID: 36979659 Free PMC article. Review.
-
High-Dose Vitamin C: Preclinical Evidence for Tailoring Treatment in Cancer Patients.Cancers (Basel). 2021 Mar 20;13(6):1428. doi: 10.3390/cancers13061428. Cancers (Basel). 2021. PMID: 33804775 Free PMC article. Review.
-
Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus spinosa, Trigno Ecotype.Cells. 2021 Nov 26;10(12):3326. doi: 10.3390/cells10123326. Cells. 2021. PMID: 34943833 Free PMC article. Review.
-
Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis.Nutrients. 2023 Apr 12;15(8):1848. doi: 10.3390/nu15081848. Nutrients. 2023. PMID: 37111066 Free PMC article.
References
-
- Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med (1985) 312(3):137–41. 10.1056/NEJM198501173120301 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

