Closing the Gap: Mechanisms of Epithelial Fusion During Optic Fissure Closure
- PMID: 33505973
- PMCID: PMC7829581
- DOI: 10.3389/fcell.2020.620774
Closing the Gap: Mechanisms of Epithelial Fusion During Optic Fissure Closure
Abstract
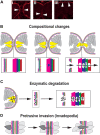
A key embryonic process that occurs early in ocular development is optic fissure closure (OFC). This fusion process closes the ventral optic fissure and completes the circumferential continuity of the 3-dimensional eye. It is defined by the coming together and fusion of opposing neuroepithelia along the entire proximal-distal axis of the ventral optic cup, involving future neural retina, retinal pigment epithelium (RPE), optic nerve, ciliary body, and iris. Once these have occurred, cells within the fused seam differentiate into components of the functioning visual system. Correct development and progression of OFC, and the continued integrity of the fused margin along this axis, are important for the overall structure of the eye. Failure of OFC results in ocular coloboma-a significant cause of childhood visual impairment that can be associated with several complex ocular phenotypes including microphthalmia and anterior segment dysgenesis. Despite a large number of genes identified, the exact pathways that definitively mediate fusion have not yet been found, reflecting both the biological complexity and genetic heterogeneity of the process. This review will highlight how recent developmental studies have become focused specifically on the epithelial fusion aspects of OFC, applying a range of model organisms (spanning fish, avian, and mammalian species) and utilizing emerging high-resolution live-imaging technologies, transgenic fluorescent models, and unbiased transcriptomic analyses of segmentally-dissected fissure tissue. Key aspects of the fusion process are discussed, including basement membrane dynamics, unique cell behaviors, and the identities and fates of the cells that mediate fusion. These will be set in the context of what is now known, and how these point the way to new avenues of research.
Keywords: EMT—epithelial to mesenchymal transition; apoptosis; basement membrane; cell polarity and adhesion; coloboma; eye development; optic fissure closure; transcriptome (RNA-seq).
Copyright © 2021 Chan, Moosajee and Rainger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

