Oral and Palatal Dentition of Axolotl Arises From a Common Tooth-Competent Zone Along the Ecto-Endodermal Boundary
- PMID: 33505974
- PMCID: PMC7829593
- DOI: 10.3389/fcell.2020.622308
Oral and Palatal Dentition of Axolotl Arises From a Common Tooth-Competent Zone Along the Ecto-Endodermal Boundary
Abstract
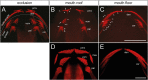
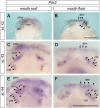
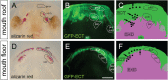
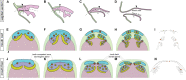
Vertebrate dentitions arise at various places within the oropharyngeal cavity including the jaws, the palate, or the pharynx. These dentitions develop in a highly organized way, where new tooth germs are progressively added adjacent to the initiator center, the first tooth. At the same time, the places where dentitions develop house the contact zones between the outer ectoderm and the inner endoderm, and this colocalization has instigated various suggestions on the roles of germ layers for tooth initiation and development. Here, we study development of the axolotl dentition, which is a complex of five pairs of tooth fields arranged into the typically tetrapod outer and inner dental arcades. By tracking the expression patterns of odontogenic genes, we reason that teeth of both dental arcades originate from common tooth-competent zones, one present on the mouth roof and one on the mouth floor. Progressive compartmentalization of these zones and a simultaneous addition of new tooth germs distinct for each prospective tooth field subsequently control the final shape and composition of the axolotl dentition. Interestingly, by following the fate of the GFP-labeled oral ectoderm, we further show that, in three out of five tooth field pairs, the first tooth develops right at the ecto-endodermal boundary. Our results thus indicate that a single tooth-competent zone gives rise to both dental arcades of a complex tetrapod dentition. Further, we propose that the ecto-endodermal boundary running through this zone should be accounted for as a potential source of instruction factors instigating the onset of the odontogenic program.
Keywords: axolotl; dental arcades; ectoderm; endoderm; initiation; patterning; tooth development.
Copyright © 2021 Soukup, Tazaki, Yamazaki, Pospisilova, Epperlein, Tanaka and Cerny.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The conundrum of pharyngeal teeth origin: the role of germ layers, pouches, and gill slits.Biol Rev Camb Philos Soc. 2022 Feb;97(1):414-447. doi: 10.1111/brv.12805. Epub 2021 Oct 13. Biol Rev Camb Philos Soc. 2022. PMID: 34647411 Free PMC article. Review.
-
Dual epithelial origin of vertebrate oral teeth.Nature. 2008 Oct 9;455(7214):795-8. doi: 10.1038/nature07304. Epub 2008 Sep 14. Nature. 2008. PMID: 18794902
-
Multiple epithelia are required to develop teeth deep inside the pharynx.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11503-11512. doi: 10.1073/pnas.2000279117. Epub 2020 May 12. Proc Natl Acad Sci U S A. 2020. PMID: 32398375 Free PMC article.
-
Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms.Biol Rev Camb Philos Soc. 2005 May;80(2):303-45. doi: 10.1017/s1464793104006682. Biol Rev Camb Philos Soc. 2005. PMID: 15921053 Review.
-
Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of the osteichthyan dentition.Evol Dev. 2006 Sep-Oct;8(5):446-57. doi: 10.1111/j.1525-142X.2006.00118.x. Evol Dev. 2006. PMID: 16925680
Cited by
-
RNA localization during early development of the axolotl.Front Cell Dev Biol. 2023 Oct 19;11:1260795. doi: 10.3389/fcell.2023.1260795. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37928901 Free PMC article.
-
Lungfish-like antero-labial tooth addition and amphibian-like enameloid-enamel transition in the coronoid of a Devonian stem actinopterygian.J Anat. 2025 Sep-Oct;247(3-4):418-441. doi: 10.1111/joa.14240. Epub 2025 Mar 13. J Anat. 2025. PMID: 40083060 Free PMC article.
-
Periderm fate and independence of tooth formation are conserved across osteichthyans.Evodevo. 2024 Oct 3;15(1):13. doi: 10.1186/s13227-024-00232-4. Evodevo. 2024. PMID: 39363199 Free PMC article.
-
The metamorphic transition of the frog mouth: from tadpole keratinized mouthparts to adult teeth.R Soc Open Sci. 2025 Sep 3;12(9):251196. doi: 10.1098/rsos.251196. eCollection 2025 Sep. R Soc Open Sci. 2025. PMID: 40904991 Free PMC article.
-
The conundrum of pharyngeal teeth origin: the role of germ layers, pouches, and gill slits.Biol Rev Camb Philos Soc. 2022 Feb;97(1):414-447. doi: 10.1111/brv.12805. Epub 2021 Oct 13. Biol Rev Camb Philos Soc. 2022. PMID: 34647411 Free PMC article. Review.
References
-
- Barlow L. A. (2000). “Taste buds in ectoderm are induced by endoderm: implications for mechanisms governing taste bud development,” in Regulatory Processes in Development, Wenner-Gren International Series, Vol. 76 eds Ollson, Jacobson C. O. (London: Portland Press; ), 185–190.
-
- Barlow L. A., Northcutt R. G. (1997). Taste buds develop autonomously from endoderm without induction by cephalic neural crest or paraxial mesoderm. Development 124 949–957. - PubMed
-
- Bordzilovskaya N. P., Dettlaff T. A., Duhon S. T., Malacinski G. M. (1989). “Developmental-stage series of axolotl embryos,” in Developmental Biology of the Axolotl, ed. Malacinski G. M. (Oxford: Oxford University Press; ), 201–219.
LinkOut - more resources
Full Text Sources
Other Literature Sources

