Biological Evaluation of Acellular Cartilaginous and Dermal Matrixes as Tissue Engineering Scaffolds for Cartilage Regeneration
- PMID: 33505975
- PMCID: PMC7829663
- DOI: 10.3389/fcell.2020.624337
Biological Evaluation of Acellular Cartilaginous and Dermal Matrixes as Tissue Engineering Scaffolds for Cartilage Regeneration
Abstract
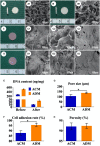
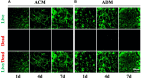
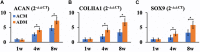
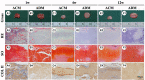
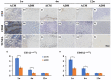
An acellular matrix (AM) as a kind of natural biomaterial is gaining increasing attention in tissue engineering applications. An acellular cartilaginous matrix (ACM) and acellular dermal matrix (ADM) are two kinds of the most widely used AMs in cartilage tissue engineering. However, there is still debate over which of these AMs achieves optimal cartilage regeneration, especially in immunocompetent large animals. In the current study, we fabricated porous ADM and ACM scaffolds by a freeze-drying method and confirmed that ADM had a larger pore size than ACM. By recolonization with goat auricular chondrocytes and in vitro culture, ADM scaffolds exhibited a higher cell adhesion rate, more homogeneous chondrocyte distribution, and neocartilage formation compared with ACM. Additionally, quantitative polymerase chain reaction (qPCR) indicated that expression of cartilage-related genes, including ACAN, COLIIA1, and SOX9, was significantly higher in the ADM group than the ACM group. Furthermore, after subcutaneous implantation in a goat, histological evaluation showed that ADM achieved more stable and matured cartilage compared with ACM, which was confirmed by quantitative data including the wet weight, volume, and contents of DNA, GAG, total collagen, and collagen II. Additionally, immunological assessment suggested that ADM evoked a low immune response compared with ACM as evidenced by qPCR and immunohistochemical analyses of CD3 and CD68, and TUNEL. Collectively, our results indicate that ADM is a more suitable AM for cartilage regeneration, which can be used for cartilage regeneration in immunocompetent large animals.
Keywords: acellular cartilaginous matrix; acellular dermal matrix; cartilage regeneration; immune responses; tissue engineering.
Copyright © 2021 Wang, Xu, Zhou, Liu and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Asawa Y., Sakamoto T., Komura M., Watanabe M., Nishizawa S., Takazawa Y., et al. (2012). Early stage foreign body reaction against biodegradable polymer scaffolds affects tissue regeneration during the autologous transplantation of tissue-engineered cartilage in the canine model. Cell Transplant. 21 1431–1442. 10.3727/096368912x640574 - DOI - PubMed
-
- Chen W., Xu Y., Li Y. Q., Jia L. T., Mo X. M., Jiang G. N., et al. (2020b). 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 382:122986 10.1016/j.cej.2019.122986 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

