The Effect of Nebivolol on Office Blood Pressure of Blacks Residing in Sub-Saharan Africa (A Pilot Study)
- PMID: 33505995
- PMCID: PMC7829216
- DOI: 10.3389/fcvm.2020.613917
The Effect of Nebivolol on Office Blood Pressure of Blacks Residing in Sub-Saharan Africa (A Pilot Study)
Abstract
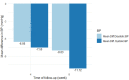
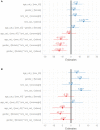
Introduction: There is substantial clinical evidence that monotherapy with beta-blockers are less effective in reducing blood pressure among hypertensive Black patients compared to Whites. The highly selective beta-1 agents like nebivolol and bisoprolol have, however, been reported to be effective in reducing blood pressure in African Americans. However, results in African Americans cannot be extrapolated to native Africans because of genetic admixture and gene-environment interaction. There is, therefore, the need for us to generate data that are applicable to Africans residing in sub-Saharan Africa. We therefore decided to evaluate the efficacy and tolerability of highly selective beta-1 agent nebivolol in hypertensive Black patients residing in sub-Saharan Africa. Materials and Methods: The nebivolol study was a multicenter, prospective, observational program among hypertensive patients with 4- and 8-week follow up which was conducted in 5 cities in Nigeria of Abuja, Calabar, Enugu, Oghara, and Port Harcourt. Dosages of nebivolol used in keeping with local prescribing information were 5 and 10 mg once daily each. The effectiveness of treatment was assessed by change from baseline in mean office systolic and diastolic blood pressures, and the proportion of patients achieving the therapeutic goal of <140/90 mmHg. Safety and tolerability of this medication were also assessed. Results: We report the results of the 140 patients studied. The mean age and body mass index were 46.9 ± 7.3 years and 22.3 ± 5.8 kg/m2, respectively, and 57.1% were female. Nebivolol reduced SBP and DBP by 7.6 and 6.6 mmHg, respectively, in 4 weeks, and by 11.1 and 8.0 mm Hg, respectively, in 8 weeks. Blood pressure control was achieved in 54.8% of the patients in 4 weeks and increased to 60.4% in 8 weeks. There was no change in metabolic profile between randomization and at 8 weeks, and erectile dysfunction occurred in 1.3% of the study population. Conclusions: Nebivolol 5 and 10 mg appear efficacious in Nigerian Africans with no negative metabolic effect and minimal side effect profile. Clinical Trial Registration: www.ClinicalTrials.gov, Study Identification: NCT03598673.
Keywords: black patients; efficacy; hypertensive; nebivolol; tolerability.
Copyright © 2021 Ojji, Ale, Shedul, Umuerri, Ejim, Alikor, Agunyenwa, Njideofor, Eze and Ansa.
Conflict of interest statement
BA was employed by the company Holo Healthcare limited, Nairobi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Joint National Committee on Prevention Detection Evaluation and Treatment of High Blood Pressure The Sixth Report of the Joint National Committee on Prevention, Detection and Treatment of High Blood Pressure. Bethsaida, MD: US Department of Health and Human Services; National High Blood Pressure Education Program; (1997).
-
- Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, et al. . for the Department of Veterans Affairs Cooperative Study Group on antihypertensive agents: age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. JAMA. (1998) 280:1168–72. 10.1001/jama.280.13.1168 - DOI - PubMed
-
- Cushman WC, Reda DJ, Perry HM, Williams D, Abdellatif M, Materson BJ. Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States. Arch Intern Med. (2000) 160:825–31. 10.1001/archinte.160.6.825 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical