New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
- PMID: 33506056
- PMCID: PMC7815414
- DOI: 10.1155/2021/6650670
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Abstract
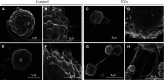
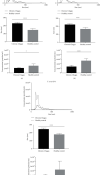
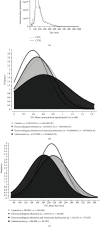
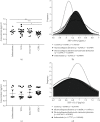
Chagas disease, a neglected tropical disease (NTD) caused by the flagellated protozoan Trypanosoma cruzi (T. cruzi), is a major public health problem. It was initially restricted to Latin America, but it is now expanding globally. Host and pathogen interactions are crucial in the establishment of disease, and since 1970, it has been known that eukaryotic cells release extracellular vesicles (EVs), which in turn have an important role in intercellular communication in physiological and pathological conditions. Our study proposed to characterize and compare circulating EVs isolated from the plasma of chronic Chagas disease (CCD) patients and controls. For this, peripheral blood was collected from patients and controls, and mononuclear cells (PBMCs) were isolated and stimulated with parasite EVs, showing that patient cells released fewer EVs than control cells. Then, after plasma separation followed by EV total shedding enrichment, the samples were subjected to ultracentrifugation to isolate the circulating EVs, which then had their size and concentration characterized by nanoparticle tracking analysis (NTA). This showed that patients had a lower concentration of circulating EVs while there were no differences in size, corroborating the in vitro data. Additionally, circulating EVs were incubated with THP-1 cells (macrophages) that, after the interaction, had their supernatant analyzed by ELISA for cytokine detection. In relation to their ability to induce cytokine production, the CCD patient EVs were able to induce a differential production of IFN-γ and IL-17 in relation to controls, with differences being more evident in earlier/less severe stages of the disease. In summary, a decreased concentration of circulating EVs associated with differential activation of the immunological system in patients with CCD is related to parasite persistence and the establishment of chronic disease. It is also a potential biomarker for monitoring disease progression.
Copyright © 2021 Rafael Pedro Madeira et al.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


Similar articles
-
Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease.Int J Mol Sci. 2025 Feb 12;26(4):1544. doi: 10.3390/ijms26041544. Int J Mol Sci. 2025. PMID: 40004010 Free PMC article.
-
Extracellular Vesicles Shed By Trypanosoma cruzi Potentiate Infection and Elicit Lipid Body Formation and PGE2 Production in Murine Macrophages.Front Immunol. 2018 Apr 27;9:896. doi: 10.3389/fimmu.2018.00896. eCollection 2018. Front Immunol. 2018. PMID: 29755471 Free PMC article.
-
Immune complexes in chronic Chagas disease patients are formed by exovesicles from Trypanosoma cruzi carrying the conserved MASP N-terminal region.Sci Rep. 2017 Mar 15;7:44451. doi: 10.1038/srep44451. Sci Rep. 2017. PMID: 28294160 Free PMC article.
-
Extracellular Vesicles in Trypanosoma cruzi Infection: Immunomodulatory Effects and Future Perspectives as Potential Control Tools against Chagas Disease.J Immunol Res. 2022 Aug 17;2022:5230603. doi: 10.1155/2022/5230603. eCollection 2022. J Immunol Res. 2022. PMID: 36033396 Free PMC article. Review.
-
Extracellular Vesicles: Potential Role in Remote Signaling and Inflammation in Trypanosoma cruzi-Triggered Disease.Front Cell Dev Biol. 2021 Dec 20;9:798054. doi: 10.3389/fcell.2021.798054. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988085 Free PMC article. Review.
Cited by
-
Extracellular vesicle and lipoprotein diagnostics (ExoLP-Dx) with membrane sensor: A robust microfluidic platform to overcome heterogeneity.Biomicrofluidics. 2024 Jul 24;18(4):041301. doi: 10.1063/5.0218986. eCollection 2024 Jul. Biomicrofluidics. 2024. PMID: 39056024 Free PMC article.
-
Host-Derived Extracellular Vesicles in Blood and Tissue Human Protozoan Infections.Microorganisms. 2023 Sep 14;11(9):2318. doi: 10.3390/microorganisms11092318. Microorganisms. 2023. PMID: 37764162 Free PMC article. Review.
-
Impact of the Extracellular Vesicles Derived From Trypanosoma cruzi: A Paradox in Host Response and Lipid Metabolism Modulation.Front Cell Infect Microbiol. 2021 Oct 28;11:768124. doi: 10.3389/fcimb.2021.768124. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34778110 Free PMC article. Review.
-
Trypanosomatid Extracellular Vesicles as Potential Immunogens for Chagas Disease.Int J Mol Sci. 2025 Feb 12;26(4):1544. doi: 10.3390/ijms26041544. Int J Mol Sci. 2025. PMID: 40004010 Free PMC article.
-
Potential of extracellular vesicles in the pathogenesis, diagnosis and therapy for parasitic diseases.J Extracell Vesicles. 2024 Aug;13(8):e12496. doi: 10.1002/jev2.12496. J Extracell Vesicles. 2024. PMID: 39113589 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

