Multicentric carpotarsal osteolysis syndrome (MCTO) with generalized high bone turnover and high serum RANKL: Response to denosumab
- PMID: 33506078
- PMCID: PMC7815641
- DOI: 10.1016/j.bonr.2021.100747
Multicentric carpotarsal osteolysis syndrome (MCTO) with generalized high bone turnover and high serum RANKL: Response to denosumab
Abstract
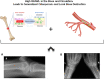
MCTO is a rare disorder, caused by mutations in the MafB gene, a negative regulator of receptor activator of nuclear factor-кB ligand (RANKL). Manifestations include carpal and tarsal osteolysis and renal failure. Pathophysiology is poorly understood, and no effective treatment is available. In this case report we describe a patient with MCTO (MafB, mutation c.206C>T, p.Ser69Leu), diagnosed at the age of 5 years. At 7 years, skeletal survey showed diffuse osteopenia. BMD was mildly reduced, and bone turnover markers increased. He was treated with denosumab, a human monoclonal RANKL inhibitor for two years. Each injection was followed by a marked reduction in C-telopeptide (CTX). Following denosumab his BMD and bone symptoms improved and the osteolysis stabilized. At the age of 13 years, osteoporosis was diagnosed using high resolution peripheral quantitative computed tomography (HRpQCT) and serum RANKL was found to be markedly increased. This initial experience suggests that the associated osteoporosis may be ameliorated by denosumab, although further study will be needed to understand the appropriate dose, frequency, and the extent of efficacy. Monitoring of CTX and bone specific alkaline phosphatase will be especially useful in this regard. Further study in other MCTO patients is also needed to determine whether high bone turnover is specific to this mutation or more common than previously appreciated. We propose a model in which osteolysis in this condition is strongly associated with the systemic osteoporosis.
Keywords: ACR, albumin to creatinine ratio; Bone turnover; CTX, C-telopeptide; Denosumab; ESKD, end stage kidney disease; HRpQCT; HRpQCT, high resolution peripheral quantitative computed tomography; MCTO; MCTO, Multicentric Carpotarsal Osteolysis Syndrome; MafB, gene v-maf musculoaponeurotic fibrosarcoma oncogene ortholog B; OPG; OPG, osteoprotegerin; Osteoporosis; RANKL; RANKL, receptor activator of nuclear factor-кB ligand.
© 2021 The Authors. Published by Elsevier Inc.
Conflict of interest statement
None. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. We declare that the manuscript is being submitted only to Bone, that it will not be submitted elsewhere, while under consideration, that it has not been published elsewhere, and, should it be published in Bone, that it will not be published elsewhere—either in similar form or verbatim—without permission of the editors. These restrictions do not apply to abstracts or to press reports of presentations at scientific meetings.
Figures
Similar articles
-
Multicentric carpotarsal osteolysis syndrome with variants of MAFB gene: a case report and literature review.Pediatr Rheumatol Online J. 2024 Mar 13;22(1):37. doi: 10.1186/s12969-024-00964-6. Pediatr Rheumatol Online J. 2024. PMID: 38481224 Free PMC article. Review.
-
Denosumab Treatment Does Not Halt Progression of Bone Lesions in Multicentric Carpotarsal Osteolysis Syndrome.JBMR Plus. 2023 Mar 9;7(5):e10729. doi: 10.1002/jbm4.10729. eCollection 2023 May. JBMR Plus. 2023. PMID: 37197321 Free PMC article.
-
Multicentric carpotarsal osteolysis syndrome is caused by only a few domain-specific mutations in MAFB, a negative regulator of RANKL-induced osteoclastogenesis.Am J Med Genet A. 2014 Sep;164A(9):2287-93. doi: 10.1002/ajmg.a.36641. Epub 2014 Jul 2. Am J Med Genet A. 2014. PMID: 24989131 Free PMC article.
-
A Familial Case of Multicentric Carpotarsal Osteolysis Syndrome and Treatment Outcome.J Pediatr Genet. 2018 Dec;7(4):174-179. doi: 10.1055/s-0038-1657760. Epub 2018 Jun 16. J Pediatr Genet. 2018. PMID: 30430035 Free PMC article.
-
Multicentric Carpotarsal Osteolysis: a Contemporary Perspective on the Unique Skeletal Phenotype.Curr Osteoporos Rep. 2023 Feb;21(1):85-94. doi: 10.1007/s11914-022-00762-7. Epub 2022 Dec 7. Curr Osteoporos Rep. 2023. PMID: 36477366 Free PMC article. Review.
Cited by
-
Multicentric Carpotarsal Osteolysis Syndrome in a Mother and Daughter with a MAFB Missense Variant and Natural History of the Disease.Mol Syndromol. 2022 Feb;13(1):50-55. doi: 10.1159/000517348. Epub 2021 Aug 27. Mol Syndromol. 2022. PMID: 35221875 Free PMC article.
-
Multicentric Carpotarsal Osteolysis Syndrome Associated Nephropathy: Novel Variants of MAFB Gene and Literature Review.J Clin Med. 2022 Jul 29;11(15):4423. doi: 10.3390/jcm11154423. J Clin Med. 2022. PMID: 35956038 Free PMC article.
-
Assessment of the concentration of osteoprotegerin and receptor activator of nuclear factor kB ligand in healthy children.Pediatr Endocrinol Diabetes Metab. 2024;30(4):183-189. doi: 10.5114/pedm.2024.146681. Pediatr Endocrinol Diabetes Metab. 2024. PMID: 39963055 Free PMC article.
-
Monogenic disorders as mimics of juvenile idiopathic arthritis.Pediatr Rheumatol Online J. 2022 Jun 18;20(1):44. doi: 10.1186/s12969-022-00700-y. Pediatr Rheumatol Online J. 2022. PMID: 35717242 Free PMC article.
-
Multicentric carpotarsal osteolysis syndrome with variants of MAFB gene: a case report and literature review.Pediatr Rheumatol Online J. 2024 Mar 13;22(1):37. doi: 10.1186/s12969-024-00964-6. Pediatr Rheumatol Online J. 2024. PMID: 38481224 Free PMC article. Review.
References
-
- AMGEN Multicenter, single-arm study to evaluate efficacy, safety, & pharmacokinetics of denosumab in children w/ OI (OI) 2020. https://clinicaltrials.gov/ct2/show/NCT02352753 Protocol Number: 20130173. ClinicalTrials.gov Identifier: NCT02352753 May. Available from:
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources