PI3K/AKT signaling drives titanium-induced angiogenic stimulus
- PMID: 33506378
- PMCID: PMC7840643
- DOI: 10.1007/s10856-020-06473-8
PI3K/AKT signaling drives titanium-induced angiogenic stimulus
Abstract
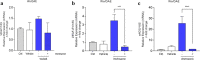
Although osseointegration and clinical success of titanium (Ti)-implanted materials depend on neovascularization in the reactional peri-implant tissue, very little has been achieved considering the Ti-molecules release on the behavior of endothelial cells. To address this issue, we challenged endothelial cells (HUVECs) with Ti-enriched medium obtained from two types of commercial titanium surfaces [presenting or not dual-acid etching (DAE)] up to 72 h to allow molecular machinery analysis. Our data show that the Ti-enriched medium provokes significant stimulus of angiogenesis-related machinery in endothelial cells by upexpressing VEGFR1, VEGFR2, VEGF, eNOS, and iNOS genes, while the PI3K/Akt signaling pathway was also significantly enhanced. As PI3K/AKT signaling was related to angiogenesis in response to vascular endothelial growth factor (VEGF), we addressed the importance of PI3K/Akt upon Ti-enriched medium responses by concomitantly treating the cells with wortmannin, a well-known PI3K inhibitor. Wortmannin suppressed the angiogenic factors, because VEGF, VEGFR1, and eNOS genes were downregulated in those cells, highlighting the importance of PI3K/AKT signaling on driving angiogenic phenotype and angiogenesis performance within the peri-implant tissue reaction. In conjunction, these data reinforce that titanium-implantable devices modify the metabolism of surrounding cells, such as endothelial cells, probably coupling osteogenesis and angiogenesis processes in peri-implant tissue and then contributing to successfully osseointegration of biomedical titanium-based devices.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Raines AL, Berger MB, Patel N, Hyzy SL, Boyan BD, Schwartz Z. VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface. J Biomed Mater Res A. 2019;107:423–33. doi: 10.1002/jbm.a.36559. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

