Collaborative Platform Trials to Fight COVID-19: Methodological and Regulatory Considerations for a Better Societal Outcome
- PMID: 33506495
- PMCID: PMC8014457
- DOI: 10.1002/cpt.2183
Collaborative Platform Trials to Fight COVID-19: Methodological and Regulatory Considerations for a Better Societal Outcome
Abstract
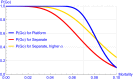
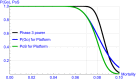
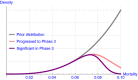
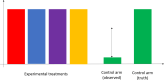
For the development of coronavirus disease 2019 (COVID-19) drugs during the ongoing pandemic, speed is of essence whereas quality of evidence is of paramount importance. Although thousands of COVID-19 trials were rapidly started, many are unlikely to provide robust statistical evidence and meet regulatory standards (e.g., because of lack of randomization or insufficient power). This has led to an inefficient use of time and resources. With more coordination, the sheer number of patients in these trials might have generated convincing data for several investigational treatments. Collaborative platform trials, comparing several drugs to a shared control arm, are an attractive solution. Those trials can utilize a variety of adaptive design features in order to accelerate the finding of life-saving treatments. In this paper, we discuss several possible designs, illustrate them via simulations, and also discuss challenges, such as the heterogeneity of the target population, time-varying standard of care, and the potentially high number of false hypothesis rejections in phase II and phase III trials. We provide corresponding regulatory perspectives on approval and reimbursement, and note that the optimal design of a platform trial will differ with our societal objective and by stakeholder. Hasty approvals may delay the development of better alternatives, whereas searching relentlessly for the single most efficacious treatment may indirectly diminish the number of lives saved as time is lost. We point out the need for incentivizing developers to participate in collaborative evidence-generation initiatives when a positive return on investment is not met.
© 2021 The Authors Clinical Pharmacology & Therapeutics © 2021 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
Similar articles
-
AGILE-ACCORD: A Randomized, Multicentre, Seamless, Adaptive Phase I/II Platform Study to Determine the Optimal Dose, Safety and Efficacy of Multiple Candidate Agents for the Treatment of COVID-19: A structured summary of a study protocol for a randomised platform trial.Trials. 2020 Jun 19;21(1):544. doi: 10.1186/s13063-020-04473-1. Trials. 2020. PMID: 32560744 Free PMC article.
-
[Repurposing of chlorpromazine in COVID-19 treatment: the reCoVery study].Encephale. 2020 Jun;46(3S):S35-S39. doi: 10.1016/j.encep.2020.04.010. Epub 2020 Apr 29. Encephale. 2020. PMID: 32387014 Free PMC article. French.
-
muLTi-Arm Therapeutic study in pre-ICu patients admitted with Covid-19-Experimental drugs and mechanisms (TACTIC-E): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jul 31;21(1):690. doi: 10.1186/s13063-020-04618-2. Trials. 2020. PMID: 32736592 Free PMC article.
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD013881. doi: 10.1002/14651858.CD013881. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jun 1;6:CD013881. doi: 10.1002/14651858.CD013881.pub2. PMID: 33734435 Free PMC article. Updated.
-
Improving resource utilisation in SLE drug development through innovative trial design.Lupus Sci Med. 2023 Jul;10(2):e000890. doi: 10.1136/lupus-2022-000890. Lupus Sci Med. 2023. PMID: 37491104 Free PMC article. Review.
Cited by
-
Clinical Evidence Informing Treatment Guidelines on Repurposed Drugs for Hospitalized Patients During the Early COVID-19 Pandemic: Corticosteroids, Anticoagulants, (Hydroxy)chloroquine.Front Public Health. 2022 Feb 18;10:804404. doi: 10.3389/fpubh.2022.804404. eCollection 2022. Front Public Health. 2022. PMID: 35252090 Free PMC article. Review.
-
Estimands and Complex Innovative Designs.Clin Pharmacol Ther. 2022 Dec;112(6):1183-1190. doi: 10.1002/cpt.2575. Epub 2022 Mar 29. Clin Pharmacol Ther. 2022. PMID: 35253205 Free PMC article. Review.
-
Innovative approaches for vaccine trials as a key component of pandemic preparedness - a white paper.Infection. 2024 Dec;52(6):2135-2144. doi: 10.1007/s15010-024-02347-1. Epub 2024 Jul 17. Infection. 2024. PMID: 39017997 Free PMC article. Review.
-
Uptake of the multi-arm multi-stage (MAMS) adaptive platform approach: a trial-registry review of late-phase randomised clinical trials.BMJ Open. 2022 Mar 10;12(3):e055615. doi: 10.1136/bmjopen-2021-055615. BMJ Open. 2022. PMID: 35273052 Free PMC article. Clinical Trial.
-
Multiplicity adjustments in parallel-group multi-arm trials sharing a control group: Clear guidance is needed.Contemp Clin Trials. 2022 Feb;113:106656. doi: 10.1016/j.cct.2021.106656. Epub 2021 Dec 11. Contemp Clin Trials. 2022. PMID: 34906747 Free PMC article. Clinical Trial.
References
-
- Coronavirus drugs trials must get bigger and more collaborative. Nature 581(7807), 120 (2020). - PubMed
-
- London, A.J. & Kimmelman, J. Against pandemic research exceptionalism. Science 368, 476–477 (2020). - PubMed
-
- Pundi, K. et al. Characteristics and strength of evidence of COVID‐19 studies registered on ClinicalTrials.gov. JAMA Intern. Med. 180, 1398–1400 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

