siRNA Therapeutics against Respiratory Viral Infections-What Have We Learned for Potential COVID-19 Therapies?
- PMID: 33506607
- PMCID: PMC7995229
- DOI: 10.1002/adhm.202001650
siRNA Therapeutics against Respiratory Viral Infections-What Have We Learned for Potential COVID-19 Therapies?
Abstract
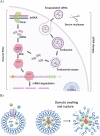
Acute viral respiratory tract infections (AVRIs) are a major burden on human health and global economy and amongst the top five causes of death worldwide resulting in an estimated 3.9 million lives lost every year. In addition, new emerging respiratory viruses regularly cause outbreaks such as SARS-CoV-1 in 2003, the "Swine flu" in 2009, or most importantly the ongoing SARS-CoV-2 pandemic, which intensely impact global health, social life, and economy. Despite the prevalence of AVRIs and an urgent need, no vaccines-except for influenza-or effective treatments were available at the beginning of the COVID-19 pandemic. However, the innate RNAi pathway offers the ability to develop nucleic acid-based antiviral drugs. siRNA sequences against conserved, essential regions of the viral genome can prevent viral replication. In addition, viral infection can be averted prophylactically by silencing host genes essential for host-viral interactions. Unfortunately, delivering siRNAs to their target cells and intracellular site of action remains the principle hurdle toward their therapeutic use. Currently, siRNA formulations and chemical modifications are evaluated for their delivery. This progress report discusses the selection of antiviral siRNA sequences, delivery techniques to the infection sites, and provides an overview of antiviral siRNAs against respiratory viruses.
Keywords: SARS-CoV-2; inhalation; nanomedicine; pulmonary delivery; respiratory virus; siRNA.
© 2021 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO , Battle against Respiratory Viruses (BRaVe) Initiative, https://www.who.int/influenza/patient_care/clinical/brave/en/ (accessed: 2020).
-
- WHO , Influenza (seasonal) Factsheet, Geneva 2016, http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed: 19 January 2021).
-
- Weekly Epidemiological Record (WER) , https://www.who.int/wer/2012/wer8747/en/ (accessed: 23 November 2012, 47, 461.
-
- Egorov A., Microbiology Independent Research journal 2018, 5, 12, 10.18527/2500-2236-2018-5-1-12-21. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

