Crosstalk between exosomes and autophagy: A review of molecular mechanisms and therapies
- PMID: 33506641
- PMCID: PMC7933923
- DOI: 10.1111/jcmm.16276
Crosstalk between exosomes and autophagy: A review of molecular mechanisms and therapies
Abstract
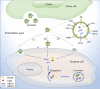
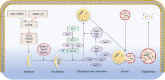
Exosomes are extracellular vesicles that primarily exist in bodily fluids such as blood. Autophagy is an intracellular degradation process, which, along with exosomes, can significantly influence human health and has therefore attracted considerable attention in recent years. Exosomes have been shown to regulate the intracellular autophagic process, which, in turn, affects the circulating exosomes. However, crosstalk between exosomal and autophagic pathways is highly complex, depends primarily on the environment, and varies greatly in different diseases. In addition, studies have demonstrated that exosomes, from specific cell, can mitigate several diseases by regulating autophagy, which can also affect the excessive release of some harmful exosomes. This phenomenon lays a theoretical foundation for the improvement of many diseases. Herein, we review the mechanisms and clinical significance of the association and regulation of exosomes and autophagy, in order to provide a new perspective for the prevention and treatment of associated diseases.
Keywords: autophagy; exosomes; mesenchymal stem cells; miRNA; molecular mechanisms; therapies.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine andJohn Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967‐978. - PubMed
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412‐9420. - PubMed
-
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

