Generation of stress fibers through myosin-driven reorganization of the actin cortex
- PMID: 33506761
- PMCID: PMC7877910
- DOI: 10.7554/eLife.60710
Generation of stress fibers through myosin-driven reorganization of the actin cortex
Erratum in
-
Correction: Generation of stress fibers through myosin-driven reorganization of the actin cortex.Elife. 2022 Apr 20;11:e79304. doi: 10.7554/eLife.79304. Elife. 2022. PMID: 35442189 Free PMC article.
Abstract
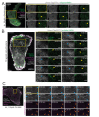
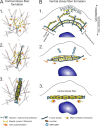
Contractile actomyosin bundles, stress fibers, govern key cellular processes including migration, adhesion, and mechanosensing. Stress fibers are thus critical for developmental morphogenesis. The most prominent actomyosin bundles, ventral stress fibers, are generated through coalescence of pre-existing stress fiber precursors. However, whether stress fibers can assemble through other mechanisms has remained elusive. We report that stress fibers can also form without requirement of pre-existing actomyosin bundles. These structures, which we named cortical stress fibers, are embedded in the cell cortex and assemble preferentially underneath the nucleus. In this process, non-muscle myosin II pulses orchestrate the reorganization of cortical actin meshwork into regular bundles, which promote reinforcement of nascent focal adhesions, and subsequent stabilization of the cortical stress fibers. These results identify a new mechanism by which stress fibers can be generated de novo from the actin cortex and establish role for stochastic myosin pulses in the assembly of functional actomyosin bundles.
Keywords: actin; cell adhesion; cell biology; cell cortex; mechanosensing; myosin; none; stress fiber.
© 2021, Lehtimäki et al.
Conflict of interest statement
JL, ER, ST No competing interests declared, PL Reviewing editor, eLife
Figures





Similar articles
-
Caldesmon controls stress fiber force-balance through dynamic cross-linking of myosin II and actin-tropomyosin filaments.Nat Commun. 2022 Oct 13;13(1):6032. doi: 10.1038/s41467-022-33688-w. Nat Commun. 2022. PMID: 36229430 Free PMC article.
-
Generation of contractile actomyosin bundles depends on mechanosensitive actin filament assembly and disassembly.Elife. 2015 Dec 10;4:e06126. doi: 10.7554/eLife.06126. Elife. 2015. PMID: 26652273 Free PMC article.
-
Myosin-18B Promotes the Assembly of Myosin II Stacks for Maturation of Contractile Actomyosin Bundles.Curr Biol. 2019 Jan 7;29(1):81-92.e5. doi: 10.1016/j.cub.2018.11.045. Epub 2018 Dec 20. Curr Biol. 2019. PMID: 30581023 Free PMC article.
-
The inner workings of stress fibers - from contractile machinery to focal adhesions and back.J Cell Sci. 2016 Apr 1;129(7):1293-304. doi: 10.1242/jcs.180927. J Cell Sci. 2016. PMID: 27037413 Review.
-
Principles of Actomyosin Regulation In Vivo.Trends Cell Biol. 2019 Feb;29(2):150-163. doi: 10.1016/j.tcb.2018.09.006. Epub 2018 Oct 29. Trends Cell Biol. 2019. PMID: 30385150 Review.
Cited by
-
Transforming growth factor-β in tumour development.Front Mol Biosci. 2022 Oct 4;9:991612. doi: 10.3389/fmolb.2022.991612. eCollection 2022. Front Mol Biosci. 2022. PMID: 36267157 Free PMC article. Review.
-
Different contractility modes control cell escape from multicellular spheroids and tumor explants.APL Bioeng. 2024 May 7;8(2):026110. doi: 10.1063/5.0188186. eCollection 2024 Jun. APL Bioeng. 2024. PMID: 38721268 Free PMC article.
-
Kinetic trapping organizes actin filaments within liquid-like protein droplets.Nat Commun. 2024 Apr 11;15(1):3139. doi: 10.1038/s41467-024-46726-6. Nat Commun. 2024. PMID: 38605007 Free PMC article.
-
Inhibiting DNA methylation as a strategy to enhance adipose-derived stem cells differentiation: Focus on the role of Akt/mTOR and Wnt/β-catenin pathways on adipogenesis.Front Cell Dev Biol. 2022 Sep 2;10:926180. doi: 10.3389/fcell.2022.926180. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120582 Free PMC article.
-
Cdc42EP3-bound septin scaffolds promote actin polymerization.J Biol Chem. 2025 Mar;301(3):108325. doi: 10.1016/j.jbc.2025.108325. Epub 2025 Feb 18. J Biol Chem. 2025. PMID: 39971161 Free PMC article.
References
-
- Baird MA, Billington N, Wang A, Adelstein RS, Sellers JR, Fischer RS, Waterman CM. Local pulsatile contractions are an intrinsic property of the myosin 2A motor in the cortical cytoskeleton of adherent cells. Molecular Biology of the Cell. 2017;28:240–251. doi: 10.1091/mbc.e16-05-0335. - DOI - PMC - PubMed
-
- Beach JR, Bruun KS, Shao L, Li D, Swider Z, Remmert K, Zhang Y, Conti MA, Adelstein RS, Rusan NM, Betzig E, Hammer JA. Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nature Cell Biology. 2017;19:85–93. doi: 10.1038/ncb3463. - DOI - PMC - PubMed
-
- Bovellan M, Romeo Y, Biro M, Boden A, Chugh P, Yonis A, Vaghela M, Fritzsche M, Moulding D, Thorogate R, Jégou A, Thrasher AJ, Romet-Lemonne G, Roux PP, Paluch EK, Charras G. Cellular control of cortical actin nucleation. Current Biology. 2014;24:1628–1635. doi: 10.1016/j.cub.2014.05.069. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

