Stem cell transplantation rescued a primary open-angle glaucoma mouse model
- PMID: 33506763
- PMCID: PMC7864631
- DOI: 10.7554/eLife.63677
Stem cell transplantation rescued a primary open-angle glaucoma mouse model
Abstract
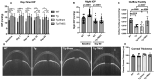
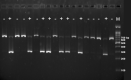
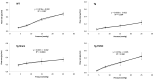
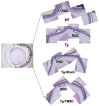
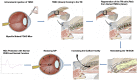
Glaucoma is a leading cause of irreversible blindness. In this study, we investigated if transplanted stem cells are able to rescue a glaucoma mouse model with transgenic myocilin Y437H mutation and explored the possible mechanisms. Human trabecular meshwork stem cells (TMSCs) were intracamerally transplanted which reduced mouse intraocular pressure, increased outflow facility, protected the retinal ganglion cells and preserved their function. TMSC transplantation also significantly increased the TM cellularity, promoted myocilin secretion from TM cells into the aqueous humor to reduce endoplasmic reticulum stress, repaired the TM tissue with extracellular matrix modulation and ultrastructural restoration. Co-culturing TMSCs with myocilin mutant TM cells in vitro promoted TMSCs differentiating into phagocytic functional TM cells. RNA sequencing revealed that TMSCs had upregulated genes related to TM regeneration and neuroprotection. Our results uncovered therapeutic potential of TMSCs for curing glaucoma and elucidated possible mechanisms by which TMSCs achieve the treatment effect.
Keywords: RNAseq; glaucoma; human; mouse; mouse model; regenerative medicine; stem cells; trabecular meshwork; transplantation.
© 2021, Xiong et al.
Conflict of interest statement
SX, AK, ST, ET, EY, PK, XX No competing interests declared, YD The University of Pittsburgh has a patent named "trabecular meshwork stem cells" with Yiqin Du as one of the inventors.
Figures







References
-
- Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Investigative Ophthalmology & Visual Science. 1981;21:714–727. - PubMed
-
- Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Science Translational Medicine. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

