Defining the optimal disease duration of early diffuse systemic sclerosis for clinical trial design
- PMID: 33506859
- PMCID: PMC8677444
- DOI: 10.1093/rheumatology/keab075
Defining the optimal disease duration of early diffuse systemic sclerosis for clinical trial design
Abstract
Objectives: Clinical trials in early diffuse cutaneous systemic sclerosis (SSc) using the modified Rodnan skin score (mRSS) as the primary outcome measure have most often been negative. We wanted to assess how the definition of disease onset (first SSc manifestation vs first non-Raynaud manifestation) and varying lengths of disease duration at trial entry as an inclusion criteria functioned. Our objective was to optimize trial inclusion criteria.
Methods: We used the prospective, observational University of Pittsburgh Scleroderma Cohort to identify early diffuse SSc patients first evaluated between 1980 and 2015. All had <3 years from first SSc (n = 481) or first non-Raynaud manifestation (n = 514) and three or more mRSS scores. We used descriptive, survival and group-based trajectory analyses to compare the different definitions of disease onset and disease duration as inclusion criteria for clinical trials.
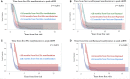
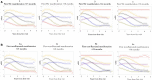
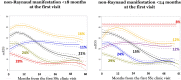
Results: There was no appreciable difference between using first SSc manifestation compared with first non-Raynaud manifestation as the definition of disease onset. Compared with other disease durations, <18 months of disease had >70% of patients fitting into trajectories with worsening cutaneous disease over 6 months of follow-up. Longer disease durations demonstrated the majority of patients with trajectories showing an improvement in mRSS (regression to the mean) over 6 months.
Conclusions: Regardless of whether the first SSc or first non-Raynaud manifestation is used to define disease onset, duration of <18 months at enrolment is preferable. A longer disease duration criterion more frequently results in regression to the mean of the mRSS score, and likely contributes to negative trial outcomes.
Keywords: clinical trial design; diffuse scleroderma; scleroderma; systemic sclerosis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Bryan C, Knight C, Black CM, Silman AJ.. Prediction of five-year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum 1999;42:2660–5. - PubMed
-
- Barnett AJ, Miller MH, Littlejohn GO.. A survival study of patients with scleroderma diagnosed over 30 years (1953-1983): the value of a simple cutaneous classification in the early stages of the disease. J Rheumatol 1988;15:276–83. - PubMed
-
- Ferri C, Valentini G, Cozzi F. et al. ; Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSS). Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–53. - PubMed
-
- Scussel-Lonzetti LJ, Raynauld JP, Roussin A. et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore) 2002;81:154–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

