Subcutaneous dosing regimens of tocilizumab in children with systemic or polyarticular juvenile idiopathic arthritis
- PMID: 33506875
- PMCID: PMC8487273
- DOI: 10.1093/rheumatology/keab047
Subcutaneous dosing regimens of tocilizumab in children with systemic or polyarticular juvenile idiopathic arthritis
Abstract
Objectives: To determine s.c. tocilizumab (s.c.-TCZ) dosing regimens for systemic JIA (sJIA) and polyarticular JIA (pJIA).
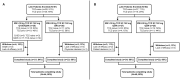
Methods: In two 52-week phase 1 b trials, s.c.-TCZ (162 mg/dose) was administered to sJIA patients every week or every 2 weeks (every 10 days before interim analysis) and to pJIA patients every 2 weeks or every 3 weeks with body weight ≥30 kg or <30 kg, respectively. Primary end points were pharmacokinetics, pharmacodynamics and safety; efficacy was exploratory. Comparisons were made to data from phase 3 trials with i.v. tocilizumab (i.v.-TCZ) in sJIA and pJIA.
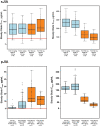
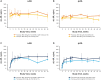
Results: Study participants were 51 sJIA patients and 52 pJIA patients aged 1-17 years who received s.c.-TCZ. Steady-state minimum TCZ concentration (Ctrough) >5th percentile of that achieved with i.v.-TCZ was achieved by 49 (96%) sJIA and 52 (100%) pJIA patients. In both populations, pharmacodynamic markers of disease were similar between body weight groups. Improvements in Juvenile Arthritis DAS-71 were comparable between s.c.-TCZ and i.v.-TCZ. By week 52, 53% of sJIA patients and 31% of pJIA patients achieved clinical remission on treatment. Safety was consistent with that of i.v.-TCZ except for injection site reactions, reported by 41.2% and 28.8% of sJIA and pJIA patients, respectively. Infections were reported in 78.4% and 69.2% of patients, respectively. Two sJIA patients died; both deaths were considered to be related to TCZ.
Conclusion: s.c.-TCZ provides exposure and risk/benefit profiles similar to those of i.v.-TCZ. S.c. administration provides an alternative administration route that is more convenient for patients and caregivers and that has potential for in-home use.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT01904292 and NCT01904279.
Keywords: autoinflammatory conditions; biologic therapies; cytokines and inflammatory mediators; inflammation; juvenile idiopathic arthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Ravelli A, Martini A.. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. - PubMed
-
- Prakken B, Albani S, Martini A.. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. - PubMed
-
- Martini A, Ravelli A, Avcin T, for the Paediatric Rheumatology International Trials Organization (PRINTO) et al.Toward new classification criteria for juvenile idiopathic arthritis: first steps, Paediatric Rheumatology International Trials Organization International Consensus. J Rheumatol 2019;46:190–7. - PubMed
-
- Guzman J, Oen K, Tucker LB. et al.The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. - PubMed
-
- Oen K, Guzman J, Dufault B, the Research in Arthritis in Canadian Children emphasizing Outcomes (ReACCh-Out) investigators et al.Health-related quality of life in an inception cohort of children with juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res 2018;70:134–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

