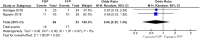Freeze-dried plasma for major trauma - Systematic review and meta-analysis
- PMID: 33507025
- PMCID: PMC7899224
- DOI: 10.1097/TA.0000000000003012
Freeze-dried plasma for major trauma - Systematic review and meta-analysis
Abstract
Background: Treatment of acute trauma coagulopathy has shifted toward rapid replacement of coagulation factors with frozen plasma (FP). There are logistic difficulties in providing FP. Freeze-dried plasma (FDP) may have logistical advantages including easier storage and rapid preparation time. This review assesses the feasibility, efficacy, and safety of FDP in trauma.
Study design and methods: Studies were searched from Medline, Embase, Cochrane Controlled Trials Register, ClinicalTrials.gov, and Google Scholar. Observational and randomized controlled trials (RCTs) assessing FDP use in trauma were included. Trauma animal models addressing FDP use were also included. Bias was assessed using validated tools. Primary outcome was efficacy, and secondary outcomes were feasibility and safety. Meta-analyses were conducted using random-effect models. Evidence was graded using Grading of Recommendations Assessment, Development, and Evaluation profile.
Results: Twelve human studies (RCT, 1; observational, 11) and 15 animal studies were included. Overall, studies demonstrated moderate risk of bias. Data from two studies (n = 119) were combined for meta-analyses for mortality and transfusion of allogeneic blood products (ABPs). For both outcomes, no difference was identified. For mortality, pooled odds ratio was 0.66 (95% confidence interval, 0.29-1.49), with I2 = 0%. Use of FDP is feasible, and no adverse events were reported. Animal data suggest similar results for coagulation and anti-inflammatory profiles for FP and FDP.
Conclusion: Human data assessing FDP use in trauma report no difference in mortality and transfusion of ABPs in patients receiving FDP compared with FP. Data from animal trauma studies report no difference in coagulation factor and anti-inflammatory profiles between FP and FDP. Results should be interpreted with caution because most studies were observational and have heterogeneous population (military and civilian trauma) and a moderate risk of bias. Well-designed prospective observational studies or, preferentially, RCTs are warranted to answer FDP's effect on laboratory (coagulation factor levels), transfusion (number of ABPs), and clinical outcomes (organ dysfunction, length of stay, and mortality).
Level of evidence: Systematic review and meta-analysis, level IV.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures
References
-
- US Centers for Disease Control and Prevention Injury Prevention and Control: Data and Statistics (WISQARS), 2014. Available at: https://webappa.cdc.gov/sasweb/ncipc/mortrate.html. Accessed June 1, 2020.
-
- Hess JR Brohi K Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–754. - PubMed
-
- Eastridge BJ, Holcomb JB, Shackelford S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion. 2019;59(S2):1423–1428. - PubMed
-
- Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes consortium. Ann Surg. 2015;261(3):586–590. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials