Intractable Coronavirus Disease 2019 (COVID-19) and Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Replication in a Chimeric Antigen Receptor-Modified T-Cell Therapy Recipient: A Case Study
- PMID: 33507235
- PMCID: PMC7929077
- DOI: 10.1093/cid/ciab072
Intractable Coronavirus Disease 2019 (COVID-19) and Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Replication in a Chimeric Antigen Receptor-Modified T-Cell Therapy Recipient: A Case Study
Abstract
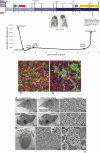
A chimeric antigen receptor-modified T-cell therapy recipient developed severe coronavirus disease 2019, intractable RNAemia, and viral replication lasting >2 months. Premortem endotracheal aspirate contained >2 × 1010 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA copies/mL and infectious virus. Deep sequencing revealed multiple sequence variants consistent with intrahost virus evolution. SARS-CoV-2 humoral and cell-mediated immunity were minimal. Prolonged transmission from immunosuppressed patients is possible.
Keywords: COVID-19; SARS-CoV-2 RNAemia; SARS-CoV-2 immune responses; SARS-CoV-2 infectivity; SARS-CoV-2 intrahost variation; severe acute respiratory syndrome coronavirus 2.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Haidar G, Garner W, Hill JA. Infections after anti-CD19 chimeric antigen receptor T-cell therapy for hematologic malignancies: timeline, prevention, and uncertainties. Curr Opin Infect Dis 2020; 33:449–57. - PubMed
-
- Wolfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

