An Analysis of Contemporary Oncology Randomized Clinical Trials From Low/Middle-Income vs High-Income Countries
- PMID: 33507236
- PMCID: PMC7844695
- DOI: 10.1001/jamaoncol.2020.7478
An Analysis of Contemporary Oncology Randomized Clinical Trials From Low/Middle-Income vs High-Income Countries
Abstract
Importance: The burden of cancer falls disproportionally on low-middle-income countries (LMICs). It is not well known how novel therapies are tested in current clinical trials and the extent to which they match global disease burden.
Objectives: To describe the design, results, and publication of oncology randomized clinical trials (RCTs) and examine the extent to which trials match global disease burden and how trial methods and results differ across economic settings.
Design, setting, and participants: In this retrospective cohort study, a literature search identified all phase 3 RCTs evaluating anticancer therapies published from 2014 to 2017. Randomized clinical trials were classified based on World Bank economic classification. Descriptive statistics were used to compare RCT design and results from high-income countries (HICs) and low/middle-income countries (LMICs). Statistical analysis was conducted in January 2020.
Main outcomes and measures: Differences in the design, results, and output of RCTs between HICs and LMICs.
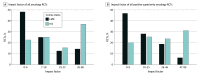
Results: The study cohort included 694 RCTs: 636 (92%) led by HICs and 58 (8%) led by LMICs. A total of 601 RCTs (87%) tested systemic therapy and 88 RCTs (13%) tested radiotherapy or surgery. The proportion of RCTs relative to global deaths was higher for breast cancer (121 RCTs [17%] and 7% of deaths) but lower for gastroesophageal cancer (38 RCTs [6%] and 14% of deaths), liver cancer (14 RCTs [2%] and 8% of deaths), pancreas cancer (14 RCTs [2%] and 5% of deaths), and cervical cancer (9 RCTs [1%] and 3% of deaths). Randomized clinical trials in HICs were more likely than those in LMICs to be funded by industry (464 [73%] vs 24 [41%]; P < .001). Studies in LMICs were smaller than those in HICs (median, 219 [interquartile range, 137-363] vs 474 [interquartile range, 262-743] participants; P < .001) and more likely to meet their primary end points (39 of 58 [67%] vs 286 of 636 [45%]; P = .001). The observed median effect size among superiority trials was larger in LMICs compared with HICs (hazard ratio, 0.62 [interquartile range, 0.54-0.76] vs 0.84 [interquartile range, 0.67-0.97]; P < .001). Studies from LMICs were published in journals with lower median impact factors than studies from HICs (7 [interquartile range, 4-21] vs 21 [interquartile range, 7-34]; P < .001). Publication bias persisted when adjusted for whether a trial was positive or negative (median impact factor: LMIC negative trial, 5 [interquartile range, 4-6] vs HIC negative trial, 18 [interquartile range, 6-26]; LMIC positive trial, 9 [interquartile range, 5-25] vs HIC positive trial, 25 [interquartile range, 10-48]; P < .001).
Conclusions and relevance: This study suggests that oncology RCTs are conducted predominantly by HICs and do not match the global burden of cancer. Randomized clinical trials from LMICs are more likely to identify effective therapies and have a larger effect size than RCTs from HICs. This study suggests that there is a funding and publication bias against RCTs led by LMICs. Policy makers, research funders, and journals need to address this issue with a range of measures including building capacity and capability in RCTs.
Conflict of interest statement
Figures

Comment in
-
Aligning Cancer Clinical Trials With Cancer Burden: Need for Greater Global Leadership, Resources, and Vision.JAMA Oncol. 2021 Mar 1;7(3):357-358. doi: 10.1001/jamaoncol.2020.7293. JAMA Oncol. 2021. PMID: 33507227 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

