Evaluation of Data Sharing After Implementation of the International Committee of Medical Journal Editors Data Sharing Statement Requirement
- PMID: 33507256
- PMCID: PMC7844597
- DOI: 10.1001/jamanetworkopen.2020.33972
Evaluation of Data Sharing After Implementation of the International Committee of Medical Journal Editors Data Sharing Statement Requirement
Abstract
Importance: The benefits of responsible sharing of individual-participant data (IPD) from clinical studies are well recognized, but stakeholders often disagree on how to align those benefits with privacy risks, costs, and incentives for clinical trialists and sponsors. The International Committee of Medical Journal Editors (ICMJE) required a data sharing statement (DSS) from submissions reporting clinical trials effective July 1, 2018. The required DSSs provide a window into current data sharing rates, practices, and norms among trialists and sponsors.
Objective: To evaluate the implementation of the ICMJE DSS requirement in 3 leading medical journals: JAMA, Lancet, and New England Journal of Medicine (NEJM).
Design, setting, and participants: This is a cross-sectional study of clinical trial reports published as articles in JAMA, Lancet, and NEJM between July 1, 2018, and April 4, 2020. Articles not eligible for DSS, including observational studies and letters or correspondence, were excluded. A MEDLINE/PubMed search identified 487 eligible clinical trials in JAMA (112 trials), Lancet (147 trials), and NEJM (228 trials). Two reviewers evaluated each of the 487 articles independently.
Exposure: Publication of clinical trial reports in an ICMJE medical journal requiring a DSS.
Main outcomes and measures: The primary outcomes of the study were declared data availability and actual data availability in repositories. Other captured outcomes were data type, access, and conditions and reasons for data availability or unavailability. Associations with funding sources were examined.
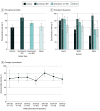
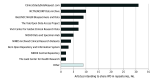
Results: A total of 334 of 487 articles (68.6%; 95% CI, 64%-73%) declared data sharing, with nonindustry NIH-funded trials exhibiting the highest rates of declared data sharing (89%; 95% CI, 80%-98%) and industry-funded trials the lowest (61%; 95% CI, 54%-68%). However, only 2 IPD sets (0.6%; 95% CI, 0.0%-1.5%) were actually deidentified and publicly available as of April 10, 2020. The remaining were supposedly accessible via request to authors (143 of 334 articles [42.8%]), repository (89 of 334 articles [26.6%]), and company (78 of 334 articles [23.4%]). Among the 89 articles declaring that IPD would be stored in repositories, only 17 (19.1%) deposited data, mostly because of embargo and regulatory approval. Embargo was set in 47.3% of data-sharing articles (158 of 334), and in half of them the period exceeded 1 year or was unspecified.
Conclusions and relevance: Most trials published in JAMA, Lancet, and NEJM after the implementation of the ICMJE policy declared their intent to make clinical data available. However, a wide gap between declared and actual data sharing exists. To improve transparency and data reuse, journals should promote the use of unique pointers to data set location and standardized choices for embargo periods and access requirements.
Conflict of interest statement
Figures

Comment in
-
Trials and Tribulations-11 Reasons Why We Need to Promote Clinical Trials Data Sharing.JAMA Netw Open. 2021 Jan 4;4(1):e2035043. doi: 10.1001/jamanetworkopen.2020.35043. JAMA Netw Open. 2021. PMID: 33507252 No abstract available.
Similar articles
-
Data Sharing and Reanalyses Among Randomized Clinical Trials Published in Surgical Journals Before and After Adoption of a Data Availability and Reproducibility Policy.JAMA Netw Open. 2022 Jun 1;5(6):e2215209. doi: 10.1001/jamanetworkopen.2022.15209. JAMA Netw Open. 2022. PMID: 35653153 Free PMC article.
-
Journal requirement for data sharing statements in clinical trials: a cross-sectional study.J Clin Epidemiol. 2024 Aug;172:111405. doi: 10.1016/j.jclinepi.2024.111405. Epub 2024 Jun 4. J Clin Epidemiol. 2024. PMID: 38838963
-
Data-sharing recommendations in biomedical journals and randomised controlled trials: an audit of journals following the ICMJE recommendations.BMJ Open. 2020 May 30;10(5):e038887. doi: 10.1136/bmjopen-2020-038887. BMJ Open. 2020. PMID: 32474433 Free PMC article.
-
Prevalence and predictors of data and code sharing in the medical and health sciences: systematic review with meta-analysis of individual participant data.BMJ. 2023 Jul 11;382:e075767. doi: 10.1136/bmj-2023-075767. BMJ. 2023. PMID: 37433624 Free PMC article.
-
Data Sharing: A New Editorial Initiative of the International Committee of Medical Journal Editors. Implications for the Editors' Network.Kardiol Pol. 2017;75(5):512-517. doi: 10.5603/KP.2017.0086. Kardiol Pol. 2017. PMID: 28530030 Review.
Cited by
-
Data Sharing and Reanalyses Among Randomized Clinical Trials Published in Surgical Journals Before and After Adoption of a Data Availability and Reproducibility Policy.JAMA Netw Open. 2022 Jun 1;5(6):e2215209. doi: 10.1001/jamanetworkopen.2022.15209. JAMA Netw Open. 2022. PMID: 35653153 Free PMC article.
-
Data-Sharing Statements Requested from Clinical Trials by Public, Environmental, and Occupational Health Journals: Cross-Sectional Study.J Med Internet Res. 2025 Feb 7;27:e64069. doi: 10.2196/64069. J Med Internet Res. 2025. PMID: 39919275 Free PMC article.
-
Data sharing statements for clinical trials: a cross-sectional survey of cardiology journals.BMJ Open. 2025 Jul 22;15(7):e097148. doi: 10.1136/bmjopen-2024-097148. BMJ Open. 2025. PMID: 40701601 Free PMC article.
-
The use of large patient databases to improve disease understanding and care.Br J Dermatol. 2022 Nov;187(5):638. doi: 10.1111/bjd.21853. Epub 2022 Sep 14. Br J Dermatol. 2022. PMID: 36104827 Free PMC article.
-
How Are Leading Research Institutions Engaging with Data Sharing Tools and Programs?AMIA Annu Symp Proc. 2024 Jan 11;2023:397-406. eCollection 2023. AMIA Annu Symp Proc. 2024. PMID: 38222386 Free PMC article.
References
-
- Institute of Medicine Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. The National Academies Press; 2015. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

