Evidence of a tolerogenic vaccine against AIDS in the Chinese macaque prefigures a potential human vaccine
- PMID: 33507389
- PMCID: PMC8036203
- DOI: 10.1007/s00705-020-04935-6
Evidence of a tolerogenic vaccine against AIDS in the Chinese macaque prefigures a potential human vaccine
Abstract
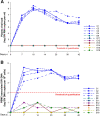
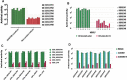
In 2006 we discovered a new type of mucosal vaccine against simian immunodeficiency virus (SIV) in Chinese macaques. Here, we review 15 years of our published work on this vaccine, which consists of inactivated SIVmac239 particles adjuvanted with Bacillus Calmette-Guérin, Lactobacillus plantarum, or Lactobacillus rhamnosus. Without adjuvant, the vaccine administered by the intragastric route induced the usual SIV-specific humoral and cellular immune responses but provided no protection against intrarectal challenge with SIVmac239. In contrast, out of 24 macaques immunized with the adjuvanted vaccine and challenged intrarectally with SIVmac239 or SIVB670, 23 were sterilely protected for up to five years, while all control macaques were infected. This protection was confirmed by an independent group from the Pasteur Institute. During the past 15 years, we have identified the mechanism of action of the vaccine and discovered that the vaccinated macaques produced a previously unrecognized class of MHC-Ib/E-restricted CD8+ T cells (which we refer to as tolerogenic CD8+ T cells) that suppressed the activation of SIV-RNA-infected CD4+ T cells and thereby inhibited the (activation-dependent) reverse transcription of the virus, which in turn prevented the establishment of SIV infection. Importantly, we discovered also that the tolerogenic CD8+ T cell subset observed in vaccinated Chinese macaques could also be found in human elite controllers, a small group of HIV-infected patients in whom these tolerogenic CD8+ T cells were shown to naturally suppress viral replication. Given that SIV and HIV require activated immune cells in which to replicate, the specific prevention of activation of SIV-RNA-containing CD4+ T cells by a tolerogenic vaccine approach offers an exciting new avenue in HIV vaccine research.
Figures



References
-
- UNAIDS report (2020).
-
- Andieu JM, Chen S, Lai C, Guo W, Lu W. Mucosal SIV vaccines comprising inactivated virus particles and bacterial adjuvants induce CD8+ T-regulatory cells that suppress SIV-positive CD4+ T-cell activation and prevent SIV infection in the macaque model. Front Immunol. 2014 doi: 10.3389/fimmu.2014.00297. - DOI - PMC - PubMed
-
- Carnathan DG, Mackela JJ, Sweata SL, Enemuoa CA, Gebrua EH, Dhadvaia P. Intragastric a dministration of Lactobacillus plantarum and AT-2-inactivated SIV does not protect Indian rhesus macaques from intra-rectal SIV challenge nor reduce virus replication after transmission. J Virol. 2018 doi: 10.1128/JVI.02030-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

