Subversion of Programed Cell Death by Poxviruses
- PMID: 33507400
- PMCID: PMC8319220
- DOI: 10.1007/82_2020_229
Subversion of Programed Cell Death by Poxviruses
Abstract
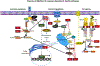
Poxviruses have been long regarded as potent inhibitors of apoptotic cell death. More recently, they have been shown to inhibit necroptotic cell death through two distinct strategies. These strategies involve either blocking virus sensing by the host pattern recognition receptor, ZBP1 (also called DAI) or by influencing receptor interacting protein kinase (RIPK)3 signal transduction by inhibition of activation of the executioner of necroptosis, mixed lineage kinase-like protein (MLKL). Vaccinia virus E3 specifically blocks ZBP1 → RIPK3 → MLKL necroptosis, leaving virus-infected cells susceptible to the TNF death-receptor signaling (e.g., TNFR1 → FADD → RIPK1 → RIPK3 → MLKL), and, potentially, TLR3 → TRIF → RIPK3 → MLKL necroptosis. While E3 restriction of necroptosis appears to be common to many poxviruses that infect vertebrate hosts, another modulatory strategy not observed in vaccinia or variola virus manifests through subversion of MLKL activation. Recently described viral mimics of MLKL in other chordopoxviruses inhibit all three modes of necroptotic cell death. As with inhibition of apoptosis, the evolution of potentially redundant viral mechanisms to inhibit programmed necroptotic cell death emphasizes the importance of this pathway in the arms race between pathogens and their hosts.
Keywords: Apoptosis; E3L; Interferon; Necroptosis; Poxviruses; Programed cell death; Z-nucleic acid-binding protein.
© 2020. Springer Nature Switzerland AG.
Figures

References
-
- Akira S, Uematsu S, and Takeuchi O (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. - PubMed
-
- Ali AN, Turner PC, Brooks MA, and Moyer RW (1994). The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology 202, 305–314. - PubMed
-
- Alsharifi M, Mullbacher A, and Regner M (2008). Interferon type I responses in primary and secondary infections. Immunol Cell Biol 86, 239–245. - PubMed
-
- Arndt WD, White SD, Johnson BP, Huynh T, Liao J, Harrington H, Cotsmire S, Kibler KV, Langland J, and Jacobs BL (2016). Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology 497, 125–135. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

