Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview
- PMID: 33507525
- PMCID: PMC7873121
- DOI: 10.1007/s40263-020-00779-5
Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview
Erratum in
-
Correction: Pharmacokinetic Characteristics of Long‑Acting Injectable Antipsychotics for Schizophrenia: An Overview.CNS Drugs. 2025 Jan;39(1):111-112. doi: 10.1007/s40263-024-01138-4. CNS Drugs. 2025. PMID: 39589630 Free PMC article. No abstract available.
Abstract
The availability of long-acting injectable (LAI) antipsychotics for the treatment of schizophrenia provides clinicians with options that deliver continuous drug exposure and may improve adherence compared with daily oral antipsychotics. However, all LAI antipsychotics have unique formulations and pharmacokinetic characteristics that have implications for medication selection, administration interval, and injection site. This review outlines key differences in drug formulations and pharmacokinetics among LAI antipsychotics. A systematic search of the PubMed database was conducted to identify physical and formulation properties and pharmacokinetic data of commercially available LAI antipsychotics, including flupentixol decanoate, fluphenazine decanoate, haloperidol decanoate, zuclopenthixol decanoate, aripiprazole monohydrate, aripiprazole lauroxil, olanzapine pamoate, paliperidone palmitate, risperidone microspheres, and risperidone polymeric microspheres. Additional information was obtained from package inserts and product monographs. Relevant data on drug properties, administration details, pharmacokinetic parameters, and oral dose equivalencies of LAI antipsychotics are summarized. Based on our analysis, formulation characteristics (e.g., vehicle medium) and administration characteristics (e.g., injection site) can affect rate of absorption and adverse effects and may factor into whether oral supplementation or an additional injection is needed. Dose adjustments may be necessary based on potential drug-drug interactions, and approximate dose equivalence with oral formulations can help inform titration when switching from oral to LAI formulations. Clinicians administering LAI antipsychotics should consider these formulation and pharmacokinetic factors to maximize clinical impact and to adjust to an individual patient's needs and treatment goals.
Conflict of interest statement
SRS is an employee of The University of Texas at Austin, College of Pharmacy, Pharmacotherapy Division. He was appointed to the Texas Health and Human Services Commission, San Antonio State Hospital and the UT Health San Antonio, Long School of Medicine; has been a consultant for Alkermes, Intra-Cellular Therapies, Genomind, Karuna, and Otsuka; has participated in speakers bureaux for Alkermes, Intra-Cellular Therapies, Neurocrine, and Teva; has served as speaker for several professional organizations; has been on the Business Development Council for the College of Psychiatric and Neurologic Pharmacists; was the Treasurer (term ended 2019) for the College of Psychiatric and Neurologic Pharmacists Foundation; and has served as an expert witness on both defendant and plaintiff sides. He has no direct stock ownership in pharmaceutical corporations. CUC has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka; served on a data safety monitoring board for Lundbeck, Rovi, Supernus, and Teva; received grant support from the Berlin Institute of Health, Janssen, the National Institute of Mental Health, the Patient-Centered Outcomes Research Institute, Takeda, and the Thrasher Foundation; and received royalties from UpToDate. He is also a stock option holder of LB Pharma. JKS, SG, MM, WH, and RV are employees and shareholders of Johnson & Johnson. EK is a former employee and shareholder of Johnson & Johnson.
Figures
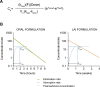


References
-
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd ed. Washington: American Psychiatric Association; 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

