Acute GVHD Diagnosis and Adjudication in a Multicenter Trial: A Report From the BMT CTN 1202 Biorepository Study
- PMID: 33507810
- PMCID: PMC8260916
- DOI: 10.1200/JCO.20.00619
Acute GVHD Diagnosis and Adjudication in a Multicenter Trial: A Report From the BMT CTN 1202 Biorepository Study
Abstract
Purpose: Accurate and reproducible methods to diagnose, grade, and report acute graft-versus-host disease (GVHD) are critical for the evaluation of therapies and biomarkers.
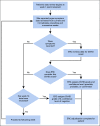
Patients and methods: The Blood and Marrow Transplant Clinical Trials Network 1202 study is an observational study of 1,709 allogeneic hematopoietic cell transplantation recipients that implemented weekly data reporting and near real-time data adjudication by an end point review committee (ERC), assigning a confidence level (confirmed, probable, possible, or negative) to the diagnosis of acute GVHD at onset.
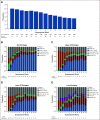
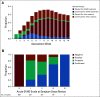
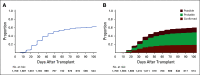
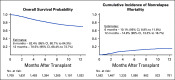
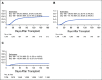
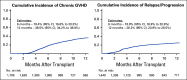
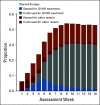
Results: During the first 100 days, symptoms consistent with GVHD developed in 90% of cases but were often determined by centers to be due to causes other than GVHD. Indeed, GVHD was under consideration in only 23% of cases at symptom onset. Diagnostic biopsies were obtained in 40% of cases, but treatment often was incongruous with biopsy findings and 10.5% of biopsies were equivocal. Importantly, more than 40% of steroid courses were started for reasons other than GVHD. The ERC modified the determination of GVHD diagnosis and/or grade in 12.3% of onset cases. The cumulative incidence of acute GVHD as reported by the centers was 62%. When the ERC adjudicated GVHD onset to be present only if the confidence level was probable or confirmed, the incidence of GVHD declined to 49%.
Conclusion: This study demonstrates that the incidence of GVHD may be overestimated at symptom onset, establishes a contemporary benchmark for acute GVHD, and suggests a structured framework for reporting and adjudication of GVHD that could be used in prospective trials.
Conflict of interest statement
Figures

References
-
- D'Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides. 2018 http://www.cibmtr.org
-
- MacMillan ML, DeFor TE, Weisdorf DJ.The best endpoint for acute GVHD treatment trials Blood 1155412–54172010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

