An abundant biliary metabolite derived from dietary omega-3 polyunsaturated fatty acids regulates triglycerides
- PMID: 33507883
- PMCID: PMC7954602
- DOI: 10.1172/JCI143861
An abundant biliary metabolite derived from dietary omega-3 polyunsaturated fatty acids regulates triglycerides
Abstract
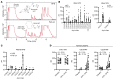
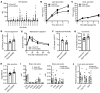
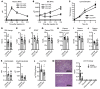
Omega-3 fatty acids from fish oil reduce triglyceride levels in mammals, yet the mechanisms underlying this effect have not been fully clarified, despite the clinical use of omega-3 ethyl esters to treat severe hypertriglyceridemia and reduce cardiovascular disease risk in humans. Here, we identified in bile a class of hypotriglyceridemic omega-3 fatty acid-derived N-acyl taurines (NATs) that, after dietary omega-3 fatty acid supplementation, increased to concentrations similar to those of steroidal bile acids. The biliary docosahexaenoic acid-containing (DHA-containing) NAT C22:6 NAT was increased in human and mouse plasma after dietary omega-3 fatty acid supplementation and potently inhibited intestinal triacylglycerol hydrolysis and lipid absorption. Supporting this observation, genetic elevation of endogenous NAT levels in mice impaired lipid absorption, whereas selective augmentation of C22:6 NAT levels protected against hypertriglyceridemia and fatty liver. When administered pharmacologically, C22:6 NAT accumulated in bile and reduced high-fat diet-induced, but not sucrose-induced, hepatic lipid accumulation in mice, suggesting that C22:6 NAT is a negative feedback mediator that limits excess intestinal lipid absorption. Thus, biliary omega-3 NATs may contribute to the hypotriglyceridemic mechanism of action of fish oil and could influence the design of more potent omega-3 fatty acid-based therapeutics.
Keywords: Endocrinology; Fatty acid oxidation; Gastroenterology; Lipoproteins.
Conflict of interest statement
Figures




Comment in
-
Triglyceride lowering by omega-3 fatty acids: a mechanism mediated by N-acyl taurines.J Clin Invest. 2021 Mar 15;131(6):e147558. doi: 10.1172/JCI147558. J Clin Invest. 2021. PMID: 33720044 Free PMC article.
References
-
- Skulas-Ray AC, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the american heart association. Circulation. 2019;140(12):E673–E691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

