Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21-65 Years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020
- PMID: 33507893
- PMCID: PMC7842810
- DOI: 10.15585/mmwr.mm7004a1
Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21-65 Years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020
Abstract
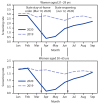
On March 19, 2020, the governor of California issued a statewide stay-at-home order to contain the spread of SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19).* The order reduced accessibility to and patient attendance at outpatient medical visits,† including preventive services such as cervical cancer screening. In-person clinic visits increased when California reopened essential businesses on June 12, 2020.§ Electronic medical records of approximately 1.5 million women served by Kaiser Permanente Southern California (KPSC), a large integrated health care system, were examined to assess cervical cancer screening rates before, during, and after the stay-at-home order. KPSC policy is to screen women aged 21-29 years every 3 years with cervical cytology alone (Papanicolaou [Pap] test); those aged 30-65 years were screened every 5 years with human papillomavirus (HPV) testing and cytology (cotesting) through July 15, 2020, and after July 15, 2020, with HPV testing alone, consistent with the latest recommendations from U.S. Preventive Services Task Force.¶ Compared with the 2019 baseline, cervical cancer screening rates decreased substantially during the stay-at-home order. Among women aged 21-29 years, cervical cytology screening rates per 100 person-months declined 78%. Among women aged 30-65 years, HPV test screening rates per 100 person-months decreased 82%. After the stay-at-home order was lifted, screening rates returned to near baseline, which might have been aided by aspects of KPSC's integrated, organized screening program (e.g., reminder systems and tracking persons lost to follow-up). As the pandemic continues, groups at higher risk for developing cervical cancers and precancers should be evaluated first. Ensuring that women receive preventive services, including cancer screening and appropriate follow-up in a safe and timely manner, remains important.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Verona, WI: Epic Health Research Network; 2020. https://ehrn.org/articles/delayed-cancer-screenings-a-second-look
-
- Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open 2020;3:e2017267. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7403918/ - PMC - PubMed
-
- Nathoo A, Weng I, Kim J, Ortuno A. Routine chronic disease screenings and oncology biomarker tests plummet during COVID-19. New York, NY: Komodo Health; 2020. https://knowledge.komodohealth.com/hubfs/white-papers/research-briefs/Ko...
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

