Fine-scale distribution of malaria mosquitoes biting or resting outside human dwellings in three low-altitude Tanzanian villages
- PMID: 33507908
- PMCID: PMC7842886
- DOI: 10.1371/journal.pone.0245750
Fine-scale distribution of malaria mosquitoes biting or resting outside human dwellings in three low-altitude Tanzanian villages
Abstract
Background: While malaria transmission in Africa still happens primarily inside houses, there is a substantial proportion of Anopheles mosquitoes that bite or rest outdoors. This situation may compromise the performance of indoor insecticidal interventions such as insecticide-treated nets (ITNs). This study investigated the distribution of malaria mosquitoes biting or resting outside dwellings in three low-altitude villages in south-eastern Tanzania. The likelihood of malaria infections outdoors was also assessed.
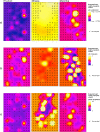
Methods: Nightly trapping was done outdoors for 12 months to collect resting mosquitoes (using resting bucket traps) and host-seeking mosquitoes (using odour-baited Suna® traps). The mosquitoes were sorted by species and physiological states. Pooled samples of Anopheles were tested to estimate proportions infected with Plasmodium falciparum parasites, estimate proportions carrying human blood as opposed to other vertebrate blood and identify sibling species in the Anopheles gambiae complex and An. funestus group. Environmental and anthropogenic factors were observed and recorded within 100 meters from each trapping positions. Generalised additive models were used to investigate relationships between these variables and vector densities, produce predictive maps of expected abundance and compare outcomes within and between villages.
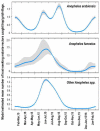
Results: A high degree of fine-scale heterogeneity in Anopheles densities was observed between and within villages. Water bodies covered with vegetation were associated with 22% higher densities of An. arabiensis and 51% lower densities of An. funestus. Increasing densities of houses and people outdoors were both associated with reduced densities of An. arabiensis and An. funestus. Vector densities were highest around the end of the rainy season and beginning of the dry seasons. More than half (14) 58.3% of blood-fed An. arabiensis had bovine blood, (6) 25% had human blood. None of the Anopheles mosquitoes caught outdoors was found infected with malaria parasites.
Conclusion: Outdoor densities of both host-seeking and resting Anopheles mosquitoes had significant heterogeneities between and within villages, and were influenced by multiple environmental and anthropogenic factors. Despite the high Anopheles densities outside dwellings, the substantial proportion of non-human blood-meals and absence of malaria-infected mosquitoes after 12 months of nightly trapping suggests very low-levels of outdoor malaria transmission in these villages.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Using a miniaturized double-net trap (DN-Mini) to assess relationships between indoor-outdoor biting preferences and physiological ages of two malaria vectors, Anopheles arabiensis and Anopheles funestus.Malar J. 2019 Aug 22;18(1):282. doi: 10.1186/s12936-019-2913-9. Malar J. 2019. PMID: 31438957 Free PMC article.
-
Wild populations of malaria vectors can mate both inside and outside human dwellings.Parasit Vectors. 2021 Oct 7;14(1):514. doi: 10.1186/s13071-021-04989-8. Parasit Vectors. 2021. PMID: 34620227 Free PMC article.
-
Analysis of the 24-h biting patterns and human exposures to malaria vectors in south-eastern Tanzania.Parasit Vectors. 2024 Oct 30;17(1):445. doi: 10.1186/s13071-024-06521-0. Parasit Vectors. 2024. PMID: 39478627 Free PMC article.
-
Mosquito feeding behavior and how it influences residual malaria transmission across Africa.Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15086-15095. doi: 10.1073/pnas.1820646116. Epub 2019 Jul 8. Proc Natl Acad Sci U S A. 2019. PMID: 31285346 Free PMC article.
-
Circadian and daily rhythms of disease vector mosquitoes.Curr Opin Insect Sci. 2024 Jun;63:101179. doi: 10.1016/j.cois.2024.101179. Epub 2024 Feb 21. Curr Opin Insect Sci. 2024. PMID: 38395256 Free PMC article. Review.
Cited by
-
Trends in malaria epidemiological factors following the implementation of current control strategies in Dangassa, Mali.Malar J. 2022 Feb 23;21(1):65. doi: 10.1186/s12936-022-04058-0. Malar J. 2022. PMID: 35197053 Free PMC article.
-
Range dynamics of Anopheles mosquitoes in Africa suggest a significant increase in the malaria transmission risk.Ecol Evol. 2024 Jul 31;14(8):e70059. doi: 10.1002/ece3.70059. eCollection 2024 Aug. Ecol Evol. 2024. PMID: 39091337 Free PMC article.
-
Evaluation of the DN-Mini (miniaturized double net) trap for sampling host-seeking Anopheles mosquitoes in malaria-endemic villages of southern Tanzania.PLoS One. 2024 Feb 14;19(2):e0294192. doi: 10.1371/journal.pone.0294192. eCollection 2024. PLoS One. 2024. PMID: 38354118 Free PMC article.
-
Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi.Malar J. 2021 Aug 26;20(1):353. doi: 10.1186/s12936-021-03885-x. Malar J. 2021. PMID: 34446033 Free PMC article.
References
-
- Matowo NS, Munhenga G, Tanner M, Coetzee M, Feringa WF, Ngowo HS, et al.: Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome open research 2017, 2 10.12688/wellcomeopenres.12617.1 - DOI - PMC - PubMed
-
- National Malaria Control Programme (Tanzania) WT, Ifakara Health Institute (Tanzania), KEMRI-Wellcome Trust (Kenya): An epidemiology profile of malaria and its control in mainland Tanzania. Report 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

