Evaluation of oral dextrose gel for prevention of neonatal hypoglycemia (hPOD): A multicenter, double-blind randomized controlled trial
- PMID: 33507929
- PMCID: PMC7842885
- DOI: 10.1371/journal.pmed.1003411
Evaluation of oral dextrose gel for prevention of neonatal hypoglycemia (hPOD): A multicenter, double-blind randomized controlled trial
Abstract
Background: Neonatal hypoglycemia is common and can cause brain injury. Buccal dextrose gel is effective for treatment of neonatal hypoglycemia, and when used for prevention may reduce the incidence of hypoglycemia in babies at risk, but its clinical utility remains uncertain.
Methods and findings: We conducted a multicenter, double-blinded, placebo-controlled randomized trial in 18 New Zealand and Australian maternity hospitals from January 2015 to May 2019. Babies at risk of neonatal hypoglycemia (maternal diabetes, late preterm, or high or low birthweight) without indications for neonatal intensive care unit (NICU) admission were randomized to 0.5 ml/kg buccal 40% dextrose or placebo gel at 1 hour of age. Primary outcome was NICU admission, with power to detect a 4% absolute reduction. Secondary outcomes included hypoglycemia, NICU admission for hypoglycemia, hyperglycemia, breastfeeding at discharge, formula feeding at 6 weeks, and maternal satisfaction. Families and clinical and study staff were unaware of treatment allocation. A total of 2,149 babies were randomized (48.7% girls). NICU admission occurred for 111/1,070 (10.4%) randomized to dextrose gel and 100/1,063 (9.4%) randomized to placebo (adjusted relative risk [aRR] 1.10; 95% CI 0.86, 1.42; p = 0.44). Babies randomized to dextrose gel were less likely to become hypoglycemic (blood glucose < 2.6 mmol/l) (399/1,070, 37%, versus 448/1,063, 42%; aRR 0.88; 95% CI 0.80, 0.98; p = 0.02) although NICU admission for hypoglycemia was similar between groups (65/1,070, 6.1%, versus 48/1,063, 4.5%; aRR 1.35; 95% CI 0.94, 1.94; p = 0.10). There were no differences between groups in breastfeeding at discharge from hospital (aRR 1.00; 95% CI 0.99, 1.02; p = 0.67), receipt of formula before discharge (aRR 0.99; 95% CI 0.92, 1.08; p = 0.90), and formula feeding at 6 weeks (aRR 1.01; 95% CI 0.93, 1.10; p = 0.81), and there was no hyperglycemia. Most mothers (95%) would recommend the study to friends. No adverse effects, including 2 deaths in each group, were attributable to dextrose gel. Limitations of this study included that most participants (81%) were infants of mothers with diabetes, which may limit generalizability, and a less reliable analyzer was used in 16.5% of glucose measurements.
Conclusions: In this placebo-controlled randomized trial, prophylactic dextrose gel 200 mg/kg did not reduce NICU admission in babies at risk of hypoglycemia but did reduce hypoglycemia. Long-term follow-up is needed to determine the clinical utility of this strategy.
Trial registration: ACTRN 12614001263684.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JH has in the past received an unrestricted research grant from Biomed Auckland, who manufacture dextrose gel. That sponsor had no role in this study, and in particular, no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures
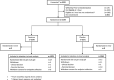

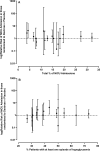
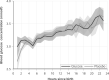
Similar articles
-
Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study).PLoS Med. 2016 Oct 25;13(10):e1002155. doi: 10.1371/journal.pmed.1002155. eCollection 2016 Oct. PLoS Med. 2016. PMID: 27780197 Free PMC article. Clinical Trial.
-
Prophylactic Oral Dextrose Gel and Neurosensory Impairment at 2-Year Follow-up of Participants in the hPOD Randomized Trial.JAMA. 2022 Mar 22;327(12):1149-1157. doi: 10.1001/jama.2022.2363. JAMA. 2022. PMID: 35315885 Free PMC article. Clinical Trial.
-
Randomised trial of neonatal hypoglycaemia prevention with oral dextrose gel (hPOD): study protocol.BMC Pediatr. 2015 Sep 16;15:120. doi: 10.1186/s12887-015-0440-6. BMC Pediatr. 2015. PMID: 26377909 Free PMC article. Clinical Trial.
-
Glucose Gel as a Treatment Strategy for Transient Neonatal Hypoglycemia.Adv Neonatal Care. 2017 Dec;17(6):470-477. doi: 10.1097/ANC.0000000000000426. Adv Neonatal Care. 2017. PMID: 28857766 Review.
-
Strategies to improve neurodevelopmental outcomes in babies at risk of neonatal hypoglycaemia.Lancet Child Adolesc Health. 2021 Jul;5(7):513-523. doi: 10.1016/S2352-4642(20)30387-4. Epub 2021 Apr 6. Lancet Child Adolesc Health. 2021. PMID: 33836151 Free PMC article. Review.
Cited by
-
Neonatal Hypoglycemia and Neurocognitive Function at School Age: A Prospective Cohort Study.J Pediatr. 2024 Sep;272:114119. doi: 10.1016/j.jpeds.2024.114119. Epub 2024 May 28. J Pediatr. 2024. PMID: 38815750
-
Are toddlers with neurosensory impairment more difficult to follow up? A secondary analysis of the hPOD follow-up study.Arch Dis Child Fetal Neonatal Ed. 2024 Oct 18;109(6):643-651. doi: 10.1136/archdischild-2023-326455. Arch Dis Child Fetal Neonatal Ed. 2024. PMID: 38604648 Clinical Trial.
-
Delivery room dextrose gel for preterm hypoglycaemia (the GEHPPI study): a randomised placebo-controlled trial.Arch Dis Child Fetal Neonatal Ed. 2025 Apr 17;110(3):319-325. doi: 10.1136/archdischild-2024-327313. Arch Dis Child Fetal Neonatal Ed. 2025. PMID: 39515988 Free PMC article. Clinical Trial.
-
Oral glucose gel in the prevention of neonatal hypoglycemia: A systematic review and meta-analysis.Medicine (Baltimore). 2023 Dec 1;102(48):e36137. doi: 10.1097/MD.0000000000036137. Medicine (Baltimore). 2023. PMID: 38050311 Free PMC article.
-
Dilemmas in parenteral glucose delivery and approach to glucose monitoring and interpretation in the neonate.J Perinatol. 2023 Sep;43(9):1200-1205. doi: 10.1038/s41372-023-01640-5. Epub 2023 Mar 24. J Perinatol. 2023. PMID: 36964206 Review.
References
-
- Hay WW Jr, Raju TNK, Higgins RD, Kalhan SC, Devaskar SU. Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. J Pediatr. 2009;155:612–7. 10.1016/j.jpeds.2009.06.044 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

