Temperature, but not excess of glycogen, regulates "in vitro" AMPK activity in muscle samples of steer carcasses
- PMID: 33507943
- PMCID: PMC7842895
- DOI: 10.1371/journal.pone.0229480
Temperature, but not excess of glycogen, regulates "in vitro" AMPK activity in muscle samples of steer carcasses
Abstract
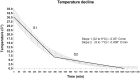
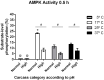
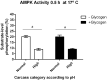
Postmortem muscle temperature affects the rate of pH decline in a linear manner from 37.5°C to 0-2°C. The pH decline is correlated with the enzymatic degradation of glycogen to lactate and this process includes the metabolic coupling between glycogenolysis and glycolysis, and that are strongly upregulated by the AMPK. In this study, we used 12 samples previously characterized by have different muscle glycogen concentration, lactate and AMPK activity, selected from 38 steers that produced high final pH (>5.9) and normal final pH (<5.8) carcasses at 24 h postmortem. Moreover, we evaluated changes in the AMPK activity in samples from both categories incubated at 37, 25, 17 and 5°C and supplemented with exogenous glycogen. Finally, we analysed if there were structural differences between polymers from both categories. Our results showed that "in vitro" enzymatic AMPK activity evaluated at both 0.5 or 24 h was greater in samples from normal then high pH categories (p <0.01), and in all temperature of incubation analysed (17, 25 and 37°C). For other hand, a greater AMPK activity were obtained in samples incubated at 17 that 25 or 37°C, in normal carcasses at both 0.5 or 24 h (p < 0.01), as also in samples from carcasses categorized as high pH, but at 24 h (p < 0.05). Interestingly, AMPK activity was totally abolished at 5°C, independent of final pH category of carcasses, and was confirmed that the incubation temperature at which the maximum activity was obtained (p < 0.01), at least in carcasses with a normal pH is at 17°C. The enzymatic AMPK activity did not change in relation to excess glycogen (p > 0.05) and we did not detect structural differences in the polymers present in samples from both categories (p > 0.05), suggesting that postmortem AMPK activity may be highly sensitive to temperature and not to in vitro changes in glycogen concentration (p > 0.05). Our results allow concluding that normal concentrations of muscle glycogen immediately at the time of slaughter (0.5 h) and an adequate cooling managing of carcasses are relevant to let an efficient glycogenolytic/glycolytic flow required for lactate accumulation and pH decline, through the postmortem AMPK signalling pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Glycolytic potential and activity of adenosine monophosphate kinase (AMPK), glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE) in steer carcasses with normal (<5.8) or high (>5.9) 24h pH determined in M. longissimus dorsi.Meat Sci. 2015 Mar;101:83-9. doi: 10.1016/j.meatsci.2014.11.008. Epub 2014 Nov 25. Meat Sci. 2015. PMID: 25462384
-
AMP-activated protein kinase (AMPK) α2 subunit mediates glycolysis in postmortem skeletal muscle.Meat Sci. 2013 Nov;95(3):536-41. doi: 10.1016/j.meatsci.2013.05.025. Epub 2013 May 25. Meat Sci. 2013. PMID: 23793092
-
Role of beta-adrenoceptor signaling and AMP-activated protein kinase in glycolysis of postmortem skeletal muscle.J Agric Food Chem. 2005 Apr 20;53(8):3235-9. doi: 10.1021/jf047913n. J Agric Food Chem. 2005. PMID: 15826083
-
Lessons to learn about postmortem metabolism using the AMPKγ3(R200Q) mutation in the pig.Meat Sci. 2011 Nov;89(3):244-50. doi: 10.1016/j.meatsci.2011.04.030. Epub 2011 May 8. Meat Sci. 2011. PMID: 21632185 Review.
-
Does AMPK bind glycogen in skeletal muscle or is the relationship correlative?Essays Biochem. 2024 Nov 18;68(3):337-347. doi: 10.1042/EBC20240006. Essays Biochem. 2024. PMID: 39192605 Free PMC article. Review.
Cited by
-
An early-postmortem metabolic comparison among three extreme acute heat stress temperature settings in chicken breast muscle.J Food Sci Technol. 2021 Dec;58(12):4823-4829. doi: 10.1007/s13197-021-05230-1. Epub 2021 Aug 14. J Food Sci Technol. 2021. PMID: 34629547 Free PMC article.
References
-
- Gallo, C. (2004). Carnes de corte oscuro en bovinos (dark cutting beef). Revista Americarne para América Latina y El Caribe (español-inglés), Año VIII (41 (Julio)), 10–13.
-
- Hargreaves A., Barrales L., Larraín R., & Zamorano L. (2003). Factores que influyen en el pH último e incidencia de corte oscuro en canales de bovino. Ciencia e Investigación Agraria, 3, 155–166.
-
- Leyva-García I.A., Figueroa-Saavedra F., Sánchez-López E., Pérez-Linares C., & Barreras-Serrano A. (2012). Impacto económico de la presencia de carne DFD en una planta de sacrificio Tipo Inspección Federal (TIF). Archivos de Medicina Veterinaria, 44, 39–42.
-
- Vidal, R., Ferrando, C., Köpfer, A., & Almuna, C. (2009). Pérdidas económicas ocasionadas por corte oscuro en ganado bovino. Resúmenes de la XXXIV Reunión Anual de la Sociedad Chilena de Producción Animal (SOCHIPA A.G.-Chile) (pp. 242–243).
-
- Apaoblaza A., Galaz A., Strobel P., Ramírez-Reveco A., Jeréz-Timaure N., & Gallo C. (2015). Glycolytic potential and activity of adenosine monophosphate kinase (AMPK), glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE) in steer carcasses with normal (< 5.8) or high (> 5.9) 24 h pH determined in M. longissimus dorsi. Meat science, 101, 83–89. 10.1016/j.meatsci.2014.11.008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

