Brain organoid formation on decellularized porcine brain ECM hydrogels
- PMID: 33507989
- PMCID: PMC7842896
- DOI: 10.1371/journal.pone.0245685
Brain organoid formation on decellularized porcine brain ECM hydrogels
Abstract
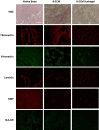
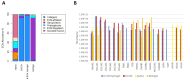
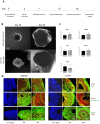
Human brain tissue models such as cerebral organoids are essential tools for developmental and biomedical research. Current methods to generate cerebral organoids often utilize Matrigel as an external scaffold to provide structure and biologically relevant signals. Matrigel however is a nonspecific hydrogel of mouse tumor origin and does not represent the complexity of the brain protein environment. In this study, we investigated the application of a decellularized adult porcine brain extracellular matrix (B-ECM) which could be processed into a hydrogel (B-ECM hydrogel) to be used as a scaffold for human embryonic stem cell (hESC)-derived brain organoids. We decellularized pig brains with a novel detergent- and enzyme-based method and analyzed the biomaterial properties, including protein composition and content, DNA content, mechanical characteristics, surface structure, and antigen presence. Then, we compared the growth of human brain organoid models with the B-ECM hydrogel or Matrigel controls in vitro. We found that the native brain source material was successfully decellularized with little remaining DNA content, while Mass Spectrometry (MS) showed the loss of several brain-specific proteins, while mainly different collagen types remained in the B-ECM. Rheological results revealed stable hydrogel formation, starting from B-ECM hydrogel concentrations of 5 mg/mL. hESCs cultured in B-ECM hydrogels showed gene expression and differentiation outcomes similar to those grown in Matrigel. These results indicate that B-ECM hydrogels can be used as an alternative scaffold for human cerebral organoid formation, and may be further optimized for improved organoid growth by further improving protein retention other than collagen after decellularization.
Conflict of interest statement
The author's have read the journal's policy and the authors of this study have the following competing interests: RS was a paid employee of VERIGRAFT AB at the time of study and HG was a paid employee of Felix Printers at the time of study. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures


References
-
- Rothenbücher TSP, Martínez-Serrano A. Human cerebral organoids and neural 3D tissues in basic research, and their application to study neurological diseases Future Neurol. Future Medicine Ltd; London, UK; 2019;14: FNL3 10.2217/fnl-2018-0043 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

