Evaluation of the practicability of a finger-stick whole-blood SARS-Cov-2 self-test adapted for the general population
- PMID: 33508007
- PMCID: PMC7842899
- DOI: 10.1371/journal.pone.0245848
Evaluation of the practicability of a finger-stick whole-blood SARS-Cov-2 self-test adapted for the general population
Abstract
Background: COVID-19 (COronaVIrus Disease 2019) is an infectious respiratory disease caused by the novel SARS-CoV-2 virus. Point of Care (POC) tests have been developed to detect specific antibodies, IgG and IgM, to SARS-CoV-2 virus in human whole blood. They need to be easily usable by the general population in order to alleviate the lockdown that many countries have initiated in response to the growing COVID-19 pandemic. A real-life study has been conducted in order to evaluate the performance of the COVID-PRESTO® POC test and the results were recently published. Even if this test showed very high sensitivity and specificity in a laboratory setting when used by trained professionals, it needs to be further evaluated for practicability when used by the general public in order to be approved by health authorities for in-home use.
Methods: 143 participants were recruited between March 2020 and April 2020 among non-medical populations in central France (nuclear plant workers, individuals attending the Orleans University Hospital vaccination clinic and Orleans University Hospital non-medical staff). Instructions for use, with or without a tutorial video, were made available to the volunteers. Two separate objectives were pursued: evaluation of the capability of participants to obtain an interpretable result, and evaluation of the users' ability to read the results.
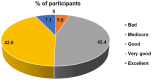
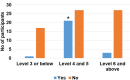
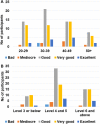
Results: 88.4% of the test users judged the instructions for use leaflet to be clear and understandable. 99.3% of the users obtained a valid result and, according to the supervisors, 92.7% of the tests were properly performed by the users. Overall, 95% of the users gave positive feedback on the COVID PRESTO® as a potential self-test. Neither age nor education had an influence.
Conclusion: COVID-PRESTO® was successfully used by an overwhelming majority of participants and its use was judged very satisfactory, therefore showing promising potential as a self-test to be used by the general population. This POC test can become an easy-to-use tool to help detect whether individuals are protected or not, particularly in the context of a second wave or a mass vaccination program.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: JPV, FS, FL, AE, DC are paid employees of Électricité de France (EDF). There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
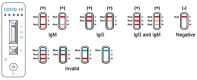
References
-
- FIND, Foundation for Innovative New Diagnostics. SARS-CoV-2 Diagnostic Pipeline. https://www.finddx.org/covid-19/pipeline/?section=immunoassays#diag_tab
-
- Food and Drug Administration. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised). https://www.fda.gov/media/135659/download
-
- European Commission. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices.
-
- European Commission. Guidelines on COVID-19 in vitro diagnostic tests and their performance. 2020. https://ec.europa.eu/info/sites/info/files/testing_kits_communication.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

