A comparison of the metabolic side-effects of the second-generation antipsychotic drugs risperidone and paliperidone in animal models
- PMID: 33508013
- PMCID: PMC7842964
- DOI: 10.1371/journal.pone.0246211
A comparison of the metabolic side-effects of the second-generation antipsychotic drugs risperidone and paliperidone in animal models
Abstract
Background: The second generation antipsychotic drugs represent the most common form of pharmacotherapy for schizophrenia disorders. It is now well established that most of the second generation drugs cause metabolic side-effects. Risperidone and its active metabolite paliperidone (9-hydroxyrisperidone) are two commonly used antipsychotic drugs with moderate metabolic liability. However, there is a dearth of preclinical data that directly compares the metabolic effects of these two drugs, using sophisticated experimental procedures. The goal of the present study was to compare metabolic effects for each drug versus control animals.
Methods: Adult female rats were acutely treated with either risperidone (0.1, 0.5, 1, 2, 6 mg/kg), paliperidone (0.1, 0.5, 1, 2, 6 mg/kg) or vehicle and subjected to the glucose tolerance test; plasma was collected to measure insulin levels to measure insulin resistance with HOMA-IR. Separate groups of rats were treated with either risperidone (1, 6 mg/kg), paliperidone (1, 6 mg/kg) or vehicle, and subjected to the hyperinsulinemic euglycemic clamp.
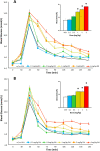
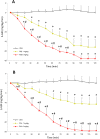
Results: Fasting glucose levels were increased by all but the lowest dose of risperidone, but only with the highest dose of paliperidone. HOMA-IR increased for both drugs with all but the lowest dose, while the three highest doses decreased glucose tolerance for both drugs. Risperidone and paliperidone both exhibited dose-dependent decreases in the glucose infusion rate in the clamp, reflecting pronounced insulin resistance.
Conclusions: In preclinical models, both risperidone and paliperidone exhibited notable metabolic side-effects that were dose-dependent. Differences between the two were modest, and most notable as effects on fasting glucose.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: WGH has received consulting fees or sat on paid advisory boards for Otsuka/Lundbeck, Newron, AlphaSights, Guidepoint, Translational Life Sciences and holds shares in Translational Life Sciences and Eli Lilly. He was additionally supported by the Jack Bell Chair in Schizophrenia. RMP has been a member of the following advisory boards in the past 3 years: Janssen, Lundbeck, and Otsuka; a member of the following speaker’s bureaus in the past 3 years: Janssen, Lundbeck, and Otsuka; and received grants from the Canadian Institutes of Health Research. All other authors declare that no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157(7):498–511. Epub 2012/08/16. 10.7326/0003-4819-157-7-201210020-00525 . - DOI - PubMed
-
- Linton D, Barr AM, Honer WG, Procyshyn RM. Antipsychotic and psychostimulant drug combination therapy in attention deficit/hyperactivity and disruptive behavior disorders: a systematic review of efficacy and tolerability. Curr Psychiatry Rep. 2013;15(5):355 10.1007/s11920-013-0355-6 . - DOI - PubMed
-
- Mela M, Hanlon-Dearman A, Ahmed AG, Rich SD, Densmore R, Reid D, et al. Treatment algorithm for the use of psychopharmacological agents in individuals prenatally exposed to alcohol and/or with diagnosis of fetal alcohol spectrum disorder (FASD). Journal of population therapeutics and clinical pharmacology = Journal de la therapeutique des populations et de la pharmacologie clinique. 2020;27(3):e1–e13. Epub 2020/08/07. 10.15586/jptcp.v27i3.681 . - DOI - PubMed
-
- Holder SD, Edmunds AL, Morgan S. Psychotic and Bipolar Disorders: Antipsychotic Drugs. FP essentials. 2017;455:23–9. Epub 2017/04/25. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

