Quantifying the dose-dependent impact of intracellular amyloid beta in a mathematical model of calcium regulation in xenopus oocyte
- PMID: 33508037
- PMCID: PMC7842920
- DOI: 10.1371/journal.pone.0246116
Quantifying the dose-dependent impact of intracellular amyloid beta in a mathematical model of calcium regulation in xenopus oocyte
Abstract
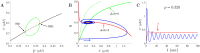
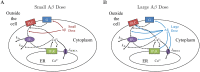
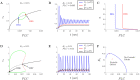
Alzheimer's disease (AD) is a devastating illness affecting over 40 million people worldwide. Intraneuronal rise of amyloid beta in its oligomeric forms (iAβOs), has been linked to the pathogenesis of AD by disrupting cytosolic Ca2+ homeostasis. However, the specific mechanisms of action are still under debate and intense effort is ongoing to improve our understanding of the crucial steps involved in the mechanisms of AβOs toxicity. We report the development of a mathematical model describing a proposed mechanism by which stimulation of Phospholipase C (PLC) by iAβO, triggers production of IP3 with consequent abnormal release of Ca2+ from the endoplasmic reticulum (ER) through activation of IP3 receptor (IP3R) Ca2+ channels. After validating the model using experimental data, we quantify the effects of intracellular rise in iAβOs on model solutions. Our model validates a dose-dependent influence of iAβOs on IP3-mediated Ca2+ signaling. We investigate Ca2+ signaling patterns for small and large iAβOs doses and study the role of various parameters on Ca2+ signals. Uncertainty quantification and partial rank correlation coefficients are used to better understand how the model behaves under various parameter regimes. Our model predicts that iAβO alter IP3R sensitivity to IP3 for large doses. Our analysis also shows that the upstream production of IP3 can influence Aβ-driven solution patterns in a dose-dependent manner. Model results illustrate and confirm the detrimental impact of iAβOs on IP3 signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures











References
-
- Association A. 2019 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. 2019;15(3):321–387. 10.1016/j.jalz.2019.01.010 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

