Hematopoietic cell-mediated dissemination of murine cytomegalovirus is regulated by NK cells and immune evasion
- PMID: 33508041
- PMCID: PMC7872266
- DOI: 10.1371/journal.ppat.1009255
Hematopoietic cell-mediated dissemination of murine cytomegalovirus is regulated by NK cells and immune evasion
Abstract
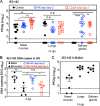
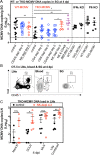
Cytomegalovirus (CMV) causes clinically important diseases in immune compromised and immune immature individuals. Based largely on work in the mouse model of murine (M)CMV, there is a consensus that myeloid cells are important for disseminating CMV from the site of infection. In theory, such dissemination should expose CMV to cell-mediated immunity and thus necessitate evasion of T cells and NK cells. However, this hypothesis remains untested. We constructed a recombinant MCMV encoding target sites for the hematopoietic specific miRNA miR-142-3p in the essential viral gene IE3. This virus disseminated poorly to the salivary gland following intranasal or footpad infections but not following intraperitoneal infection in C57BL/6 mice, demonstrating that dissemination by hematopoietic cells is essential for specific routes of infection. Remarkably, depletion of NK cells or T cells restored dissemination of this virus in C57BL/6 mice after intranasal infection, while dissemination occurred normally in BALB/c mice, which lack strong NK cell control of MCMV. These data show that cell-mediated immunity is responsible for restricting MCMV to hematopoietic cell-mediated dissemination. Infected hematopoietic cells avoided cell-mediated immunity via three immune evasion genes that modulate class I MHC and NKG2D ligands (m04, m06 and m152). MCMV lacking these 3 genes spread poorly to the salivary gland unless NK cells were depleted, but also failed to replicate persistently in either the nasal mucosa or salivary gland unless CD8+ T cells were depleted. Surprisingly, CD8+ T cells primed after intranasal infection required CD4+ T cell help to expand and become functional. Together, our data suggest that MCMV can use both hematopoietic cell-dependent and -independent means of dissemination after intranasal infection and that cell mediated immune responses restrict dissemination to infected hematopoietic cells, which are protected from NK cells during dissemination by viral immune evasion. In contrast, viral replication within mucosal tissues depends on evasion of T cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

