Nonpungent N-AVAM Capsaicin Analogues and Cancer Therapy
- PMID: 33508189
- PMCID: PMC10442063
- DOI: 10.1021/acs.jmedchem.0c01679
Nonpungent N-AVAM Capsaicin Analogues and Cancer Therapy
Abstract
Capsaicin displays robust growth-inhibitory activity in multiple human cancers. However, the feasibility of capsaicin as a clinically relevant anticancer drug is hampered by its adverse side effects. This concern has led to extensive research focused on the isolation and synthesis of second-generation nonpungent capsaicin analogues with potent antineoplastic activity. A major class of nonpungent capsaicin-like compounds belongs to the N-acyl-vanillylamide (N-AVAM) derivatives of capsaicin (hereafter referred as N-AVAM capsaicin analogues). This perspective discusses the isolation of N-AVAM capsaicin analogues from natural sources as well as their synthesis by chemical and enzymatic methods. The perspective describes the pharmacokinetic properties and anticancer activity of N-AVAM capsaicin analogues. The signaling pathways underlying the growth-inhibitory effects of N-AVAM capsaicin analogues have also been highlighted. It is hoped that the insights obtained in this perspective will facilitate the synthesis of a second generation of N-AVAM capsaicin analogues with improved stability and growth-suppressive activity in human cancer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










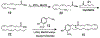
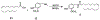

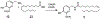
Similar articles
-
Anticancer Activity of Region B Capsaicin Analogs.J Med Chem. 2023 Apr 13;66(7):4294-4323. doi: 10.1021/acs.jmedchem.2c01594. Epub 2023 Mar 31. J Med Chem. 2023. PMID: 37000154 Free PMC article. Review.
-
Infringement of the barriers of cancer via dietary phytoconstituents capsaicin through novel drug delivery system.Curr Drug Deliv. 2016;13(1):27-39. doi: 10.2174/1567201812666150603151250. Curr Drug Deliv. 2016. PMID: 26036845 Review.
-
Capsaicin and its analogues: structure-activity relationship study.Curr Med Chem. 2013;20(21):2661-72. doi: 10.2174/0929867311320210004. Curr Med Chem. 2013. PMID: 23627937 Review.
-
Comparison of the effects of pelargonic acid vanillylamide and capsaicin on human vanilloid receptors.Phytother Res. 2013 Jul;27(7):1048-53. doi: 10.1002/ptr.4817. Epub 2012 Sep 7. Phytother Res. 2013. PMID: 22961689
-
Anticancer Properties of Capsaicin Against Human Cancer.Anticancer Res. 2016 Mar;36(3):837-43. Anticancer Res. 2016. PMID: 26976969 Review.
Cited by
-
One-carbon homologation of alkenes.Nature. 2025 Jul;643(8070):130-138. doi: 10.1038/s41586-025-09159-9. Epub 2025 May 20. Nature. 2025. PMID: 40393510 Free PMC article.
-
Anticancer Activity of Region B Capsaicin Analogs.J Med Chem. 2023 Apr 13;66(7):4294-4323. doi: 10.1021/acs.jmedchem.2c01594. Epub 2023 Mar 31. J Med Chem. 2023. PMID: 37000154 Free PMC article. Review.
-
1,2,3-Triazole Tethered Hybrid Capsaicinoids as Antiproliferative Agents Active against Lung Cancer Cells (A549).ACS Omega. 2022 Sep 1;7(36):32078-32100. doi: 10.1021/acsomega.2c03325. eCollection 2022 Sep 13. ACS Omega. 2022. PMID: 36119972 Free PMC article.
References
-
- Blair NT; Carvacho I; Chaudhuri D; Clapham DE; DeCaen P; Delling M; Doerner JF; Fan L; Ha K; Jordt SE; Julius D; Kahle KT; Liu B; McKemy D; Nilius B; Oancea E; Owsianik G; Riccio A; Sah R; Stotz SC; Tian J; Tong D; Van den Eynde C; Vriens J; Wu L-J; Xu H; Yue L; Zhang X; Zhu MX Transient receptor potential channels. (version 2019.4) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide to Pharmacology CITE 2019, DOI: 10.2218/gtopdb/F78/2019.4. - DOI
-
- Caterina MJ; Schumacher MA; Tominaga M; Rosen TA; Levine JD; Julius D The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

