Biomarker-guided tuberculosis preventive therapy (CORTIS): a randomised controlled trial
- PMID: 33508224
- PMCID: PMC7907670
- DOI: 10.1016/S1473-3099(20)30914-2
Biomarker-guided tuberculosis preventive therapy (CORTIS): a randomised controlled trial
Abstract
Background: Targeted preventive therapy for individuals at highest risk of incident tuberculosis might impact the epidemic by interrupting transmission. We tested performance of a transcriptomic signature of tuberculosis (RISK11) and efficacy of signature-guided preventive therapy in parallel, using a hybrid three-group study design.
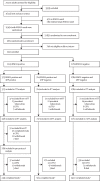
Methods: Adult volunteers aged 18-59 years were recruited at five geographically distinct communities in South Africa. Whole blood was sampled for RISK11 by quantitative RT-PCR assay from eligible volunteers without HIV, recent previous tuberculosis (ie, <3 years before screening), or comorbidities at screening. RISK11-positive participants were block randomised (1:2; block size 15) to once-weekly, directly-observed, open-label isoniazid and rifapentine for 12 weeks (ie, RISK11 positive and 3HP positive), or no treatment (ie, RISK11 positive and 3HP negative). A subset of eligible RISK11-negative volunteers were randomly assigned to no treatment (ie, RISK11 negative and 3HP negative). Diagnostic discrimination of prevalent tuberculosis was tested in all participants at baseline. Thereafter, prognostic discrimination of incident tuberculosis was tested in the untreated RISK11-positive versus RISK11-negative groups, and treatment efficacy in the 3HP-treated versus untreated RISK11-positive groups, during active surveillance through 15 months. The primary endpoint was microbiologically confirmed pulmonary tuberculosis. The primary outcome measures were risk ratio [RR] for tuberculosis of RISK11-positive to RISK11-negative participants, and treatment efficacy. This trial is registered with ClinicalTrials.gov, NCT02735590.
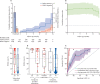
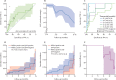
Findings: 20 207 volunteers were screened, and 2923 participants were enrolled, including RISK11-positive participants randomly assigned to 3HP (n=375) or no 3HP (n=764), and 1784 RISK11-negative participants. Cumulative probability of prevalent or incident tuberculosis disease was 0·066 (95% CI 0·049 to 0·084) in RISK11-positive (3HP negative) participants and 0·018 (0·011 to 0·025) in RISK11-negative participants (RR 3·69, 95% CI 2·25-6·05) over 15 months. Tuberculosis prevalence was 47 (4·1%) of 1139 versus 14 (0·78%) of 1984 in RISK11-positive compared with RISK11-negative participants, respectively (diagnostic RR 5·13, 95% CI 2·93 to 9·43). Tuberculosis incidence over 15 months was 2·09 (95% CI 0·97 to 3·19) vs 0·80 (0·30 to 1·30) per 100 person years in RISK11-positive (3HP-negative) participants compared with RISK11-negative participants (cumulative incidence ratio 2·6, 95% CI 1·2 to 5·9). Serious adverse events related to 3HP included one hospitalisation for seizures (unintentional isoniazid overdose) and one death of unknown cause (possibly temporally related). Tuberculosis incidence over 15 months was 1·94 (95% CI 0·35 to 3·50) versus 2·09 (95% CI 0·97 to 3·19) per 100 person-years in 3HP-treated RISK11-positive participants compared with untreated RISK11-positive participants (efficacy 7·0%, 95% CI -145 to 65).
Interpretation: The RISK11 signature discriminated between individuals with prevalent tuberculosis, or progression to incident tuberculosis, and individuals who remained healthy, but provision of 3HP to signature-positive individuals after exclusion of baseline disease did not reduce progression to tuberculosis over 15 months.
Funding: Bill and Melinda Gates Foundation, South African Medical Research Council.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Transcriptomic signatures have a place in short-term prediction of incident tuberculosis.Lancet Infect Dis. 2021 Mar;21(3):299-300. doi: 10.1016/S1473-3099(20)30980-4. Epub 2021 Jan 25. Lancet Infect Dis. 2021. PMID: 33508223 No abstract available.
References
-
- WHO . World Health Organization; Geneva: 2014. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting.https://apps.who.int/iris/bitstream/handle/10665/135617/WHO_HTM_TB_2014....
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

