Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition
- PMID: 33508232
- PMCID: PMC7933824
- DOI: 10.1016/j.cell.2021.01.002
Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition
Abstract
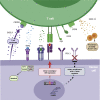
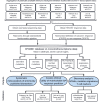
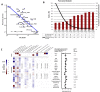
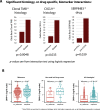
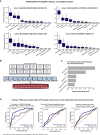
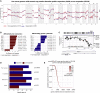
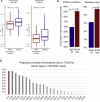
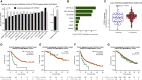
Checkpoint inhibitors (CPIs) augment adaptive immunity. Systematic pan-tumor analyses may reveal the relative importance of tumor-cell-intrinsic and microenvironmental features underpinning CPI sensitization. Here, we collated whole-exome and transcriptomic data for >1,000 CPI-treated patients across seven tumor types, utilizing standardized bioinformatics workflows and clinical outcome criteria to validate multivariable predictors of CPI sensitization. Clonal tumor mutation burden (TMB) was the strongest predictor of CPI response, followed by total TMB and CXCL9 expression. Subclonal TMB, somatic copy alteration burden, and histocompatibility leukocyte antigen (HLA) evolutionary divergence failed to attain pan-cancer significance. Dinucleotide variants were identified as a source of immunogenic epitopes associated with radical amino acid substitutions and enhanced peptide hydrophobicity/immunogenicity. Copy-number analysis revealed two additional determinants of CPI outcome supported by prior functional evidence: 9q34 (TRAF2) loss associated with response and CCND1 amplification associated with resistance. Finally, single-cell RNA sequencing (RNA-seq) of clonal neoantigen-reactive CD8 tumor-infiltrating lymphocytes (TILs), combined with bulk RNA-seq analysis of CPI-responding tumors, identified CCR5 and CXCL13 as T-cell-intrinsic markers of CPI sensitivity.
Keywords: CXCL9; biomarkers; checkpoint inhibitors; clonal TMB; immunogenicity; immunotherapy; meta-analysis; mutation; neoantigen.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.L. has a patent on indel burden and CPI response pending and outside of the submitted work, speaker fees from Roche tissue diagnostics, research funding from CRUK TDL/Ono/LifeArc alliance, and a consulting role with Monopteros Therapeutics. S.T. has received speaking fees from Roche, AstraZeneca, Novartis, and Ipsen. S.T. has the following patents filed: Indel mutations as a therapeutic target and predictive biomarker PCTGB2018/051892 and PCTGB2018/051893 and Clear Cell Renal Cell Carcinoma Biomarkers P113326GB. S.Q. reports personal fees and employment with Achilles Therapeutics (where he is CSO) outside of the submitted work. J.L.R. consults for Achilles Therapeutics. N.M. has received consultancy fees and has stock options in Achilles Therapeutics. N.M. holds European patents relating to targeting neoantigens (PCT/EP2016/ 059401), identifying patient response to immune checkpoint blockade (PCT/ EP2016/071471), determining HLA LOH (PCT/GB2018/052004), and predicting survival rates of patients with cancer (PCT/GB2020/050221). C.A. receives research salary from AstraZeneca and is an AstraZeneca Fellow and acting study physician on the MERMAID-1 study. C.A. holds pending patents in methods to detect tumor recurrence (PCT/GB2017/053289). C.A. and C.S. declare patent PCT/US2017/028013 for methods to detect lung cancer. C.A. has received speaker fees from Novartis, Roche Diagnostics, Bristol Myers Squibb, and AstraZeneca and was an advisory board member for AstraZeneca. M.D.H. has stock and other ownership interests in Shattuck Labs, Immunai, and Arcus Biosciences; reports honoraria from AstraZeneca and Bristol-Myers Squibb; has a consulting or advisory role with Bristol-Myers Squibb, Merck, Genentech/Roche, AstraZeneca, Nektar, Syndax, Mirati Therapeutics, Shattuck Labs, Immunai, Blueprint Medicines, Achilles Therapeutics, and Arcus Biosciences; receives research funding from Bristol-Myers Squibb (Inst); has patents, royalties, and other intellectual property (a patent has been filed by Memorial Sloan Kettering [PCT/US2015/062208] for the use of TMB for prediction of immunotherapy efficacy, which is licensed to Personal Genome Diagnostics); and receives travel and accommodation expense reimbursement from AstraZeneca, Bristol-Myers Squibb, and Eli Lilly. J.L. reports personal fees from Eisai, GlaxoSmithKline, Kymab, Roche/Genentech, Secarna, Pierre Fabre, and EUSA Pharma and grants and personal fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, and Novartis outside of the submitted work. C. Swanton acknowledges grant support from Pfizer, AstraZeneca, Bristol-Myers Squibb, Roche-Ventana, Boehringer-Ingelheim, Archer Dx (collaboration in minimal residual disease sequencing technologies), and Ono Pharmaceutical; is an AstraZeneca advisory board member and chief investigator for the MeRmaiD1 clinical trial; has consulted for Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol-Myers Squibb, Celgene, Amgen, AstraZeneca, Illumina, Genentech, Roche-Ventana, GRAIL, Medicxi, Bicycle Therapeutics, and the Sarah Cannon Research Institute; has stock options in Apogen Biotechnologies, Epic Bioscience, and GRAIL; and has stock options and is co-founder of Achilles Therapeutics. C.S. holds European patents relating to assay technology to detect tumor recurrence (PCT/GB2017/053289), targeting neoantigens (PCT/EP2016/059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), predicting survival rates of patients with cancer (PCT/GB2020/050221), and identifying patients who respond to cancer treatment (PCT/GB2018/051912), as well as a US patent relating to detecting tumor mutations (PCT/US2017/28013) and both a European and US patent related to identifying insertion/deletion mutation targets (PCT/GB2018/051892). S.R.H. is co-founder of Tetramershop and PokeAcell. D.B. reports personal fees from NanoString, outside this work, and he has a patent PCT/GB2020/050221 issued on methods for cancer prognostication.
Figures





Comment in
-
Strength in numbers: predicting response to checkpoint inhibitors from large clinical datasets.Cell. 2021 Feb 4;184(3):571-573. doi: 10.1016/j.cell.2021.01.008. Epub 2021 Jan 27. Cell. 2021. PMID: 33508231
-
Immunotherapy biomarkers: the long and winding road.Nat Rev Clin Oncol. 2021 Jun;18(6):323-324. doi: 10.1038/s41571-021-00498-w. Nat Rev Clin Oncol. 2021. PMID: 33727676 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- 211179/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- 24956/CRUK_/Cancer Research UK/United Kingdom
- 30025/CRUK_/Cancer Research UK/United Kingdom
- 29911/CRUK_/Cancer Research UK/United Kingdom
- P30 CA008748/CA/NCI NIH HHS/United States
- C50947/A18176/CRUK_/Cancer Research UK/United Kingdom
- MR/M009033/1/MRC_/Medical Research Council/United Kingdom
- 22246/CRUK_/Cancer Research UK/United Kingdom
- FC001202/WT_/Wellcome Trust/United Kingdom
- C416/A25145/CRUK_/Cancer Research UK/United Kingdom
- 28990/CRUK_/Cancer Research UK/United Kingdom
- 20466/CRUK_/Cancer Research UK/United Kingdom
- 18176/CRUK_/Cancer Research UK/United Kingdom
- 25253/CRUK_/Cancer Research UK/United Kingdom
- FC001169/WT_/Wellcome Trust/United Kingdom
- MR/P014712/2/MRC_/Medical Research Council/United Kingdom
- MC_PC_17179/MRC_/Medical Research Council/United Kingdom
- 17786/CRUK_/Cancer Research UK/United Kingdom
- C33499/A20265/CRUK_/Cancer Research UK/United Kingdom
- MR/P014712/1/MRC_/Medical Research Council/United Kingdom
- C69256/A30194/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

