SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement
- PMID: 33508241
- PMCID: PMC7834976
- DOI: 10.1016/S2468-1253(21)00024-8
SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement
Abstract
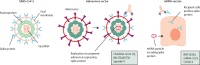
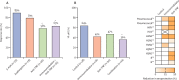
SARS-CoV-2 has caused a global health crisis and mass vaccination programmes provide the best opportunity for controlling transmission and protecting populations. Despite the impressive clinical trial results of the BNT162b2 (Pfizer/BioNTech), ChAdOx1 nCoV-19 (Oxford/AstraZeneca), and mRNA-1273 (Moderna) vaccines, important unanswered questions remain, especially in patients with pre-existing conditions. In this position statement endorsed by the British Society of Gastroenterology Inflammatory Bowel Disease (IBD) section and IBD Clinical Research Group, we consider SARS-CoV-2 vaccination strategy in patients with IBD. The risks of SARS-CoV-2 vaccination are anticipated to be very low, and we strongly support SARS-CoV-2 vaccination in patients with IBD. Based on data from previous studies with other vaccines, there are conceptual concerns that protective immune responses to SARS-CoV-2 vaccination may be diminished in some patients with IBD, such as those taking anti-TNF drugs. However, the benefits of vaccination, even in patients treated with anti-TNF drugs, are likely to outweigh these theoretical concerns. Key areas for further research are discussed, including vaccine hesitancy and its effect in the IBD community, the effect of immunosuppression on vaccine efficacy, and the search for predictive biomarkers of vaccine success.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
SARS-CoV-2 vaccination in immunosuppressed patients with inflammatory bowel disease: should our approach change?Lancet Gastroenterol Hepatol. 2021 Jul;6(7):528-529. doi: 10.1016/S2468-1253(21)00184-9. Epub 2021 May 26. Lancet Gastroenterol Hepatol. 2021. PMID: 34048723 Free PMC article. No abstract available.
-
SARS-CoV-2 vaccination for patients with inflammatory bowel disease - Authors' reply.Lancet Gastroenterol Hepatol. 2021 Jul;6(7):523-524. doi: 10.1016/S2468-1253(21)00194-1. Lancet Gastroenterol Hepatol. 2021. PMID: 34119033 Free PMC article. No abstract available.
-
SARS-CoV-2 vaccination for patients with inflammatory bowel disease.Lancet Gastroenterol Hepatol. 2021 Jul;6(7):523. doi: 10.1016/S2468-1253(21)00148-5. Lancet Gastroenterol Hepatol. 2021. PMID: 34119034 Free PMC article. No abstract available.
References
-
- El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2–10. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

