Enhanced accessibility and hydrophobicity of amyloidogenic intermediates of the β2-microglobulin D76N mutant revealed by high-pressure experiments
- PMID: 33508321
- PMCID: PMC7950326
- DOI: 10.1016/j.jbc.2021.100333
Enhanced accessibility and hydrophobicity of amyloidogenic intermediates of the β2-microglobulin D76N mutant revealed by high-pressure experiments
Abstract
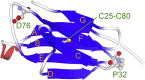
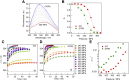
β2-Microglobulin (β2m) is the causative protein of dialysis-related amyloidosis. Its unfolding mainly proceeds along the pathway of NC →UC ⇄ UT, whereas refolding follows the UT → IT (→NT) →NC pathway, in which N, I, and U are the native, intermediate, and unfolded states, respectively, with the Pro32 peptidyl-prolyl bond in cis or trans conformation as indicated by the subscript. It is noted that the IT state is a putative amyloidogenic precursor state. Several aggregation-prone variants of β2m have been reported to date. One of these variants is D76N β2m, which is a naturally occurring amyloidogenic mutant. To elucidate the molecular mechanisms contributing to the enhanced amyloidogenicity of the mutant, we investigated the equilibrium and kinetic transitions of pressure-induced folding/unfolding equilibria in the wild type and D76N mutant by monitoring intrinsic tryptophan and 1-anilino-8-naphthalene sulfonate fluorescence. An analysis of kinetic data revealed that the different folding/unfolding behaviors of the wild type and D76N mutant were due to differences in the activation energy between the unfolded and the intermediate states as well as stability of the native state, leading to more rapid accumulation of IT state for D76N in the refolding process. In addition, the IT state was found to assume more hydrophobic nature. These changes induced the enhanced amyloidogenicity of the D76N mutant and the distinct pathogenic symptoms of patients. Our results suggest that the stabilization of the native state will be an effective approach for suppressing amyloid fibril formation of this mutant.
Keywords: amyloid; biophysics; fluorescence; high-pressure experiment; mutant; protein folding; β2-microglobulin.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Iwata K., Matsuura T., Sakurai K., Nakagawa A., Goto Y. High-resolution crystal structure of β2-microglobulin formed at pH 7.0. J. Biochem. 2007;142:413–419. - PubMed
-
- Naiki H., Hashimoto N., Suzuki S., Kiumura H., Nakakuki K., Gejyo F. Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro. Amyloid. 1997;4:223–232.
-
- Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T., Cohen A.S., Schmid K. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem. Biophys. Res. Commun. 1985;129:701–706. - PubMed
-
- Kad N.M., Myers S.L., Smith D.P., Smith D.A., Radford S.E., Thomson N.H. Hierarchical assembly of beta2-microglobulin amyloid in vitro revealed by atomic force microscopy. J. Mol. Biol. 2003;330:785–797. - PubMed
-
- Corazza A., Pettirossi F., Viglino P., Verdone G., Garcia J., Dumy P., Giorgetti S., Mangione P., Raimondi S., Stoppini M., Bellotti V., Esposito G. Properties of some variants of human beta2-microglobulin and amyloidogenesis. J. Biol. Chem. 2004;279:9176–9189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

