SARS-CoV-2 infection in patients with autoimmune hepatitis
- PMID: 33508378
- PMCID: PMC7835076
- DOI: 10.1016/j.jhep.2021.01.021
SARS-CoV-2 infection in patients with autoimmune hepatitis
Abstract
Background & aims: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) continues to have a devastating impact across the globe. However, little is known about the disease course in patients with autoimmune hepatitis (AIH).
Methods: Data for patients with AIH and SARS-CoV-2 infection were combined from 3 international reporting registries and outcomes were compared to those in patients with chronic liver disease of other aetiology (non-AIH CLD) and to patients without liver disease (non-CLD).
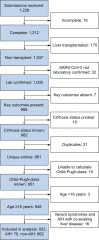
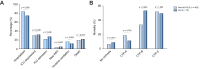
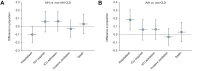
Results: Between 25th March and 24th October 2020, data were collected for 932 patients with CLD and SARS-CoV-2 infection including 70 with autoimmune hepatitis (AIH). Fifty-eight (83%) patients with AIH were taking ≥1 immunosuppressive drug. There were no differences in rates of major outcomes between patients with AIH and non-AIH CLD, including hospitalization (76% vs. 85%; p = 0.06), intensive care unit admission (29% vs. 23%; p = 0.240), and death (23% vs. 20%; p = 0.643). Factors associated with death within the AIH cohort included age (odds ratio [OR] 2.16/10 years; 1.07-3.81), and Child-Pugh class B (OR 42.48; 4.40-409.53), and C (OR 69.30; 2.83-1694.50) cirrhosis, but not use of immunosuppression. Propensity score matched (PSM) analysis comparing patients with AIH with non-AIH CLD demonstrated no increased risk of adverse outcomes including death (+3.2%; -9.2%-15.7%). PSM analysis of patients with AIH vs. non-CLD (n = 769) demonstrated increased risk of hospitalization with AIH (+18.4%; 5.6-31.2%), but equivalent risk of all other outcomes including death (+3.2%; -9.1%-15.6%).
Conclusion: Patients with AIH were not at increased risk of adverse outcomes despite immunosuppressive treatment compared to other causes of CLD and to matched cases without liver disease.
Lay summary: Little is known about the outcomes of COVID-19 in patients with autoimmune hepatitis (AIH), a rare chronic inflammatory liver disease. This study combines data from 3 large registries to describe the course of COVID-19 in this patient group. We show that AIH patients do not appear to have an increased risk of death from COVID-19 compared to patients with other forms of liver disease and compared to patients without liver disease, despite the use of medications which suppress the immune system.
Keywords: COVID-19; SARS-CoV-2; autoimmune hepatitis; coronavirus; immunosuppression; liver disease.
Copyright © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors do not have any conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
SARS-CoV-2-specific immunity in immunosuppressed COVID-19 convalescents with autoimmune hepatitis.J Hepatol. 2021 Dec;75(6):1506-1509. doi: 10.1016/j.jhep.2021.07.012. Epub 2021 Jul 17. J Hepatol. 2021. PMID: 34284030 Free PMC article. No abstract available.
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Https://coronavirus.jhu.edu/map.html.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

