Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma
- PMID: 33509020
- PMCID: PMC8290324
- DOI: 10.1089/thy.2020.0322
Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma
Abstract
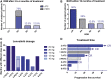
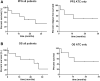
Background: Anaplastic thyroid carcinoma (ATC) and metastatic poorly differentiated thyroid carcinomas (PDTCs) are rare aggressive malignancies with poor overall survival (OS) despite extensive multimodal therapy. These tumors are highly proliferative, with frequently increased tumor mutational burden (TMB) compared with differentiated thyroid carcinomas, and elevated programmed death ligand 1 (PD-L1) levels. These tumor properties implicate responsiveness to antiangiogenic and antiproliferative multikinase inhibitors such as lenvatinib, and immune checkpoint inhibitors such as pembrolizumab. Patients and Methods: In a retrospective study, we analyzed six patients with metastatic ATC and two patients with PDTC, who received a combination therapy of lenvatinib and pembrolizumab. Lenvatinib was started at 14-24 mg daily and combined with pembrolizumab at a fixed dose of 200 mg every three weeks. Maximum treatment duration with this combination was 40 months, and 3 of 6 ATC patients are still on therapy. Patient tumors were characterized by whole-exome sequencing and PD-L1 expression levels (tumor proportion score [TPS] 1-90%). Results: Best overall response (BOR) within ATCs was 66% complete remissions (4/6 CR), 16% stable disease (1/6 SD), and 16% progressive disease (1/6 PD). BOR within PDTCs was partial remission (PR 2/2). The median progression-free survival was 17.75 months for all patients, and 16.5 months for ATCs, with treatment durations ranging from 1 to 40 months (1, 4, 11, 15, 19, 25, 27, and 40 months). Grade III/IV toxicities developed in 4 of 8 patients, requiring dose reduction/discontinuation of lenvatinib. The median OS was 18.5 months, with three ATC patients being still alive without relapse (40, 27, and 19 months) despite metastatic disease at the time of treatment initiation (UICC and stage IVC). All patients with long-term (>2 years) or complete responses (CRs) had either increased TMB or a PD-L1 TPS >50%. Conclusions: Our results implicate that the combination of lenvatinib and pembrolizumab might be safe and effective in patients with ATC/PDTC and can result in complete and long-term remissions. The combination treatment is now being systematically examined in a phase II clinical trial (Anaplastic Thyroid Carcinoma Lenvatinib Pembrolizumab [ATLEP]) in ATC/PDTC patients.
Keywords: ATC; PDTC; anaplastic thyroid cancer; lenvatinib; pembrolizumab; poorly differentiated thyroid cancer.
Conflict of interest statement
C.D. received honoraria and travel costs for consultancy role at Eisai, AbbVie, and Gilead. M.K. received honoraria and travel costs for consultancy role at Eisai GmbH. A.Z. received honoraria and travel costs for consultancy role at Eisai, J&J, and Merck and participates in the Keynote 158 trial.
Figures
References
-
- Wendler J, Kroiss M, Gast K, Kreissl MC, Allelein S, Lichtenauer U, Blaser R, Spitzweg C, Fassnacht M, Schott M, Fuhrer D, Tiedje V. 2016. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany. Eur J Endocrinol 175:521–529 - PubMed
-
- Prasongsook N, Kumar A, Chintakuntlawar AV, Foote RL, Kasperbauer J, Molina J, Garces Y, Ma D, Wittich MAN, Rubin J, Richardson R, Morris J, Hay I, Fatourechi V, McIver B, Ryder M, Thompson G, Grant C, Richards M, Sebo TJ, Rivera M, Suman V, Jenkins SM, Smallridge RC, Bible KC. 2017. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab 102:4506–4514 - PubMed
-
- Cabanillas ME, Zafereo M, Gunn GB, Ferrarotto R. 2016. Anaplastic thyroid carcinoma: treatment in the age of molecular targeted therapy. J Oncol Pract 12:511–518 - PubMed
-
- Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, Rubinstein JC, Choi M, Kiss N, Nelson-Williams C, Mane S, Rimm DL, Prasad ML, Hoog A, Zedenius J, Larsson C, Korah R, Lifton RP, Carling T. 2015. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet 24:2318–2329 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
