Performance Characteristics of BinaxNOW COVID-19 Antigen Card for Screening Asymptomatic Individuals in a University Setting
- PMID: 33509809
- PMCID: PMC8092740
- DOI: 10.1128/JCM.03282-20
Performance Characteristics of BinaxNOW COVID-19 Antigen Card for Screening Asymptomatic Individuals in a University Setting
Abstract
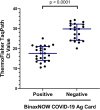
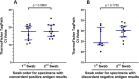
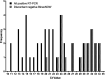
We compared the performance of the Abbott BinaxNOW COVID-19 antigen card to that of a standard reverse transcription-PCR (RT-PCR) assay (Thermo Fisher TaqPath COVID-19 Combo kit) for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2,645 asymptomatic students presenting for screening at the University of Utah. SARS-CoV-2 RNA was detected in 1.7% of the study participants by RT-PCR. BinaxNOW identified 24 infections but missed 21 infections that were detected by RT-PCR. The analytical sensitivity (positive agreement) and analytical specificity (negative agreement) for the BinaxNOW were 53.3% and 100%, respectively, compared to the RT-PCR assay. The median cycle threshold (CT ) value in the specimens that had concordant positive BinaxNOW antigen results was significantly lower than that of specimens that were discordant (CT of 17.6 versus 29.6; P < 0.001). In individuals with presumably high viral loads (CT of <23.0), a 95.8% positive agreement was observed between the RT-PCR assay and BinaxNOW. Due to the possibility of false-negative results, caution must be taken when utilizing rapid antigen testing for screening asymptomatic individuals.
Keywords: BinaxNOW COVID-19 antigen card; SARS-CoV-2; asymptomatic screening; rapid antigen tests.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, Spijker R, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group . 2020. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database Syst Rev 8:CD013705. 10.1002/14651858.CD013705. - DOI - PMC - PubMed
-
- US Food and Drug Administration. 22 December 2020, accession date. Individual EUAs for antigen diagnostic tests for SARS-CoV-2. US Food and Drug Administration, Silver Spring, MD. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em....
-
- US Department of Health and Human Services. 2020. Guidance for PREP Act coverage for COVID-19 screening tests at nursing homes, assisted-living facilities, long-term-care facilities, and other congregate facilities. US Department of Health and Human Services, Washington, DC. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/.... Accessed 19 December 2020.
-
- Abbott Diagnostics Scarborough, Inc. 2020. BinaxNOW COVID-19 Ag card product insert. Abbott Diagnostics Scarborough, Inc, Scarborough, ME.
-
- US Department of Health and Human Services. 2020. Trump administration will deploy 150 million rapid tests in 2020. US Department of Health and Human Services, Washington, DC. https://www.hhs.gov/about/news/2020/08/27/trump-administration-will-depl.... Accessed 19 December 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

