Retinoblastoma: What the Neuroradiologist Needs to Know
- PMID: 33509920
- PMCID: PMC8041013
- DOI: 10.3174/ajnr.A6949
Retinoblastoma: What the Neuroradiologist Needs to Know
Abstract
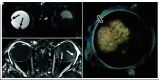
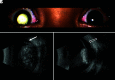
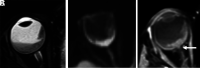
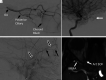
Retinoblastoma is the most common primary intraocular tumor of childhood. Accurate diagnosis at an early stage is important to maximize patient survival, globe salvage, and visual acuity. Management of retinoblastoma is individualized based on the presenting clinical and imaging features of the tumor, and a multidisciplinary team is required to optimize patient outcomes. The neuroradiologist is a key member of the retinoblastoma care team and should be familiar with characteristic diagnostic and prognostic imaging features of this disease. Furthermore, with the adoption of intra-arterial chemotherapy as a standard of care option for globe salvage therapy in many centers, the interventional neuroradiologist may play an active role in retinoblastoma treatment. In this review, we discuss the clinical presentation of retinoblastoma, ophthalmic imaging modalities, neuroradiology imaging features, and current treatment options.
© 2021 by American Journal of Neuroradiology.
Figures


References
-
- Shields JA, Leonard BC, Michelson JB, et al. . B-scan ultrasonography in the diagnosis of atypical retinoblastomas. Can J Ophthalmol 1976;11:42–51 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
