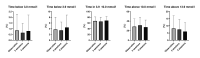Time spent in hypoglycemia is comparable when the same amount of exercise is performed 5 or 2 days weekly: a randomized crossover study in people with type 1 diabetes
- PMID: 33509935
- PMCID: PMC7845672
- DOI: 10.1136/bmjdrc-2020-001919
Time spent in hypoglycemia is comparable when the same amount of exercise is performed 5 or 2 days weekly: a randomized crossover study in people with type 1 diabetes
Abstract
Introduction: People with type 1 diabetes are recommended to exercise regularly. However, limited evidence exists on how frequency and duration of exercise affect the risk of hypoglycemia. The study aimed to compare the percentage of time spent in hypoglycemia between two 5-day periods with different frequency and duration of physical activity.
Research design and methods: In this outpatient randomized crossover study, 26 participants aged 18-65 years with type 1 diabetes for ≥2 years and insulin pump use for ≥1 year were included. After a 7-day observation period, participants completed two 5-day intervention periods separated by a washout period of at least 14 days. One period included five exercise sessions on 5 consecutive days (5S), each consisting of 4 min of resistance training and 30 min of aerobic exercise. Another period included two exercise sessions on 2 days with at least 2 days in between (2S), each consisting of 10 min of resistance training and 75 min of aerobic exercise. During each period, participants performed in total 150 min of aerobic exercise and 20 min of resistance training and wore continuous glucose monitors (Dexcom G6) and accelerometers (ActiGraph wGT3X-BT).
Results: Twenty insulin pump-treated adults (10 women) with type 1 diabetes completed the study. The baseline median (range) age was 48 (24-64) years, glycated hemoglobin 55 (44-66) mmol/mol, diabetes duration 24 (8-57) years, and body mass index 28.4 (22.3-35.8) kg/m2. No differences were observed between 5S and 2S in the percentage (mean±SD) of time spent below 3.9 mmol/L (3.5%±2.8% vs 4.5%±4.2%, p=0.28), time spent in 3.9-10.0 mmol/L (65.3%±15.0% vs 68.5%±13.6%, p=0.31), time spent above 10.0 mmol/L (31.2%±16.4% vs 27.3%±14.5%, p=0.15), mean glucose (8.7±1.3 mmol/L vs 8.5±1.2 mmol/L, p=0.33) and glycemic variability (35.8%±5.3% vs 35.8%±6.6%, p=0.97).
Conclusions: Time spent in hypoglycemia was comparable between the two 5-day periods with different duration and frequency of physical activity.
Trial registration number: NCT04089462.
Keywords: diabetes mellitus; exercise; hypoglycemia; type 1.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IIKS has received speaker grants from Roche Diabetes Care and Rubin Medical. SS has served on the continuous glucose monitoring advisory board of Roche Diabetes Care. KN serves as adviser for Medtronic, Abbott and Novo Nordisk A/S, owns shares in Novo Nordisk A/S, has received research grants from Novo Nordisk A/S, Zealand Pharma, Medtronic and Roche Diabetes Care, and has received fees for speaking from Medtronic, Roche Diabetes Care, Rubin Medical, Sanofi, Novo Nordisk A/S, Zealand Pharma and Dexcom. AGR declares no duality of interest associated with his contribution to this manuscript.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous