Expansion sequencing: Spatially precise in situ transcriptomics in intact biological systems
- PMID: 33509999
- PMCID: PMC7900882
- DOI: 10.1126/science.aax2656
Expansion sequencing: Spatially precise in situ transcriptomics in intact biological systems
Abstract
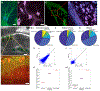
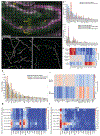
Methods for highly multiplexed RNA imaging are limited in spatial resolution and thus in their ability to localize transcripts to nanoscale and subcellular compartments. We adapt expansion microscopy, which physically expands biological specimens, for long-read untargeted and targeted in situ RNA sequencing. We applied untargeted expansion sequencing (ExSeq) to the mouse brain, which yielded the readout of thousands of genes, including splice variants. Targeted ExSeq yielded nanoscale-resolution maps of RNAs throughout dendrites and spines in the neurons of the mouse hippocampus, revealing patterns across multiple cell types, layer-specific cell types across the mouse visual cortex, and the organization and position-dependent states of tumor and immune cells in a human metastatic breast cancer biopsy. Thus, ExSeq enables highly multiplexed mapping of RNAs from nanoscale to system scale.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




Comment in
-
Expanding views of the transcriptome.Nat Methods. 2021 Apr;18(4):332. doi: 10.1038/s41592-021-01119-1. Nat Methods. 2021. PMID: 33828267 No abstract available.
References
-
- Crosetto N, Bienko M, van Oudenaarden A, Spatially resolved transcriptomics and beyond. Nat. Rev. Genet 16, 57–66 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
- P50 HG005550/HG/NHGRI NIH HHS/United States
- R01 MH114031/MH/NIMH NIH HHS/United States
- 21143/CRUK_/Cancer Research UK/United Kingdom
- HHSN261201500003C/CA/NCI NIH HHS/United States
- T32 EB001680/EB/NIBIB NIH HHS/United States
- UF1 NS107697/NS/NINDS NIH HHS/United States
- R01 EB024261/EB/NIBIB NIH HHS/United States
- HHSN261201500003I/CA/NCI NIH HHS/United States
- R01 MH110932/MH/NIMH NIH HHS/United States
- R01 DA045549/DA/NIDA NIH HHS/United States
- U19 MH114821/MH/NIMH NIH HHS/United States
- 20544/CRUK_/Cancer Research UK/United Kingdom
- R01 NS102727/NS/NINDS NIH HHS/United States
- RM1 HG008525/HG/NHGRI NIH HHS/United States
- R01 MH103910/MH/NIMH NIH HHS/United States
- T32 CA009172/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

