Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke
- PMID: 33510055
- PMCID: PMC8328755
- DOI: 10.4103/1673-5374.306093
Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke
Abstract
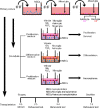
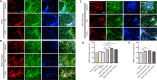
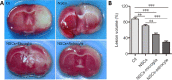
Transplantation of neural stem cells (NSCs) can protect neurons in animal stroke models; however, their low rates of survival and neuronal differentiation limit their clinical application. Glial niches, an important location of neural stem cells, regulate survival, proliferation and differentiation of neural stem cells. However, the effects of activated glial cells on neural stem cells remain unclear. In the present study, we explored the effects of activated astrocytes and microglia on neural stem cells in vitro stroke models. We also investigated the effects of combined transplantation of neural stem cells and glial cells after stroke in rats. In a Transwell co-culture system, primary cultured astrocytes, microglia or mixed glial cells were exposed to glutamate or H2O2 and then seeded in the upper inserts, while primary neural stem cells were seeded in the lower uncoated wells and cultured for 7 days. Our results showed that microglia were conducive to neurosphere formation and had no effects on apoptosis within neurospheres, while astrocytes and mixed glial cells were conducive to neurosphere differentiation and reduced apoptosis within neurospheres, regardless of their pretreatment. In contrast, microglia and astrocytes induced neuronal differentiation of neural stem cells in differentiation medium, regardless of their pretreatment, with an exception of astrocytes pretreated with H2O2. Rat models of ischemic stroke were established by occlusion of the middle cerebral artery. Three days later, 5 × 105 neural stem cells with microglia or astrocytes were injected into the right lateral ventricle. Neural stem cell/astrocyte-treated rats displayed better improvement of neurological deficits than neural stem cell only-treated rats at 4 days after cell transplantation. Moreover, neural stem cell/microglia-, and neural stem cell/astrocyte-treated rats showed a significant decrease in ischemic volume compared with neural stem cell-treated rats. These findings indicate that microglia and astrocytes exert different effects on neural stem cells, and that co-transplantation of neural stem cells and astrocytes is more conducive to the recovery of neurological impairment in rats with ischemic stroke. The study was approved by the Animal Ethics Committee of Tongji University School of Medicine, China (approval No. 2010-TJAA08220401) in 2010.
Keywords: astrocytes; glutamate; microglia; neural stem cells; neurogenesis; neurons; peroxide; repair; stroke.
Conflict of interest statement
None
Figures





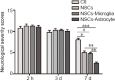
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. - PubMed
-
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. - PubMed
-
- Chen B, Gao XQ, Yang CX, Tan SK, Sun ZL, Yan NH, Pang YG, Yuan M, Chen GJ, Xu GT, Zhang K, Yuan QL. Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res. 2009;1284:1–11. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

